Page 183 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 183
174 CONTAMINANT SORPTION TO SOILS AND NATURAL SOLIDS
20
1, 2, 3-Trichlorobenzene (24°C)
(mg/L)
15
150
Apparent Solute Solubility 100 2, 4, 4'-PCB (24°C)
10
(µg/L)
5 2, 4, 5, 2', 5'-PCB (24°C)
p, p' -DDT (23°C)
0
0 20 40 60 80 100
Concentration of Polyacrylic Acid, X (mg/L)
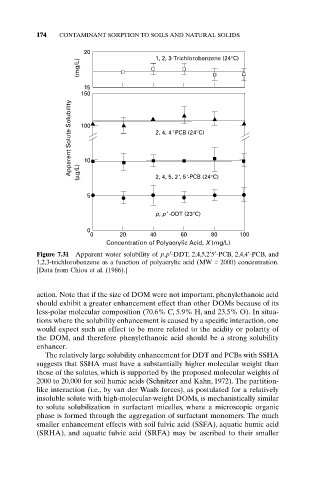
Figure 7.31 Apparent water solubility of p,p¢-DDT, 2,4,5,2¢5¢-PCB, 2,4,4¢-PCB, and
1,2,3-trichlorobenzene as a function of polyacrylic acid (MW = 2000) concentration.
[Data from Chiou et al. (1986).]
action. Note that if the size of DOM were not important, phenylethanoic acid
should exhibit a greater enhancement effect than other DOMs because of its
less-polar molecular composition (70.6% C, 5.9% H, and 23.5% O). In situa-
tions where the solubility enhancement is caused by a specific interaction, one
would expect such an effect to be more related to the acidity or polarity of
the DOM, and therefore phenylethanoic acid should be a strong solubility
enhancer.
The relatively large solubility enhancement for DDT and PCBs with SSHA
suggests that SSHA must have a substantially higher molecular weight than
those of the solutes, which is supported by the proposed molecular weights of
2000 to 20,000 for soil humic acids (Schnitzer and Kahn, 1972). The partition-
like interaction (i.e., by van der Waals forces), as postulated for a relatively
insoluble solute with high-molecular-weight DOMs, is mechanistically similar
to solute solubilization in surfactant micelles, where a microscopic organic
phase is formed through the aggregation of surfactant monomers. The much
smaller enhancement effects with soil fulvic acid (SSFA), aquatic humic acid
(SRHA), and aquatic fulvic acid (SRFA) may be ascribed to their smaller