Page 95 - Petroleum Geology
P. 95
74
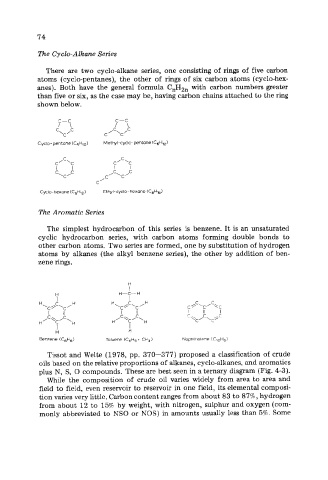
The Cyclo-Alkane Series
There are two cyclo-alkane series, one consisting of rings of five carbon
atoms (cyclo-pentanes), the other of rings of six carbon atoms (cyclo-hex-
anes). Both have the general formula C,H,, with carbon numbers greater
than five or six, as the case may be, having carbon chains attached to the ring
shown below.
Cycio- pentane (C,H,,) Methyl-cyclo- pentane (C6Hl2)
Cydo- hexane (C6HI2) Ethyl - cyclo - hexane (C8H16)
The Aromatic Series
The simplest hydrocarbon of this series is benzene. It is an unsaturated
cyclic hydrocarbon series, with carbon atoms forming double bonds to
other carbon atoms. Two series are formed, one by substitution of hydrogen
atoms by alkanes (the alkyl benzene series), the other by addition of ben-
zene rings.
H
I
H H-C-H
I I
H
Benzene (C,H,) Toluene (C6H5. CH3) Naphthalene (C,oH,)
Tissot and Welte (1978, pp. 370-377) proposed a classification of crude
oils based on the relative proportions of alkanes, cyclo-alkanes, and aromatics
plus N, S, 0 compounds. These are best seen in a ternary diagram (Fig. 4-3).
While the composition of crude oil varies widely from area to area and
field to field, even reservoir to reservoir in one field, its elemental composi-
tion varies very little. Carbon content ranges from about 83 to 87%, hydrogen
from about 12 to 15% by weight, with nitrogen, sulphur and oxygen (com-
monly abbreviated to NSO or NOS) in amounts usually less than 5%. Some