Page 96 - Petroleum Geology
P. 96
75
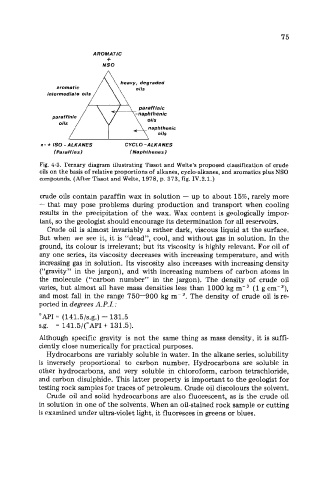
AROMATIC
+
NSO
aromatic
n- + IS0 -ALKANES CYCLO -ALKANES
(Paraffins) (Naphthenes)
Fig. 4-3. Ternary diagram illustrating Tissot and Welte’s proposed classification of crude
oils on the basis of relative proportions of alkanes, cyclo-alkanes, and aromatics plus NSO
compounds. (After Tissot and Welte, 1978, p. 373, fig. IV.2.1.)
crude oils contain paraffin wax in solution - up to about 15%, rarely more
- that may pose problems during production and transport when cooling
results in the precipitation of the wax. Wax content is geologically impor-
tant, so the geologist should encourage its determination for all reservoirs.
Crude oil is almost invariably a rather dark, viscous liquid at the surface.
But when we see it, it is “dead”, cool, and without gas in solution. In the
ground, its colour is irrelevant; but its viscosity is highly relevant. For oil of
any one series, its viscosity decreases with increasing temperature, and with
increasing gas in solution. Its viscosity also increases with increasing density
(“gravity” in the jargon), and with increasing numbers of carbon atoms in
the molecule (“carbon number” in the jargon). The density of crude oil
varies, but almost all have mass densities less than 1000 kg m-3 (1 g cm- 3),
and most fall in the range 750-900 kg m- ’. The density of crude oil is re-
ported in degrees A.P.I. :
“API = (141.5/s.g.) - 131.5
s.g. = 141.5/(”API + 131.5).
Although specific gravity is not the same thing as mass density, it is suffi-
ciently close numerically for practical purposes.
Hydrocarbons are variably soluble in water. In the alkane series, solubility
is inversely proportional to carbon number. Hydrocarbons are soluble in
other hydrocarbons, and very soluble in chloroform, carbon tetrachloride,
and carbon disulphide. This latter property is important to the geologist for
testing rock samples for traces of petroleum. Crude oil discolours the solvent.
Crude oil and solid hydrocarbons are also fluorescent, as is the crude oil
in solution in one of the solvents. When an oil-stained rock sample or cutting
is examined under ultra-violet light, it fluoresces in greens or blues.