Page 108 - Petrophysics 2E
P. 108
PROBLEMS 81
H H H H
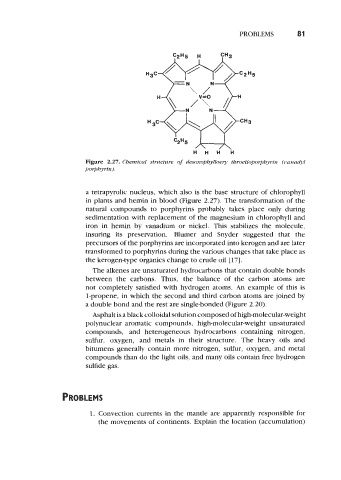
Figure 2.27. Chemical structure of desoxophylloe y throetioporphyrin (uanadyl
porphyrin).
a tetrapyrolic nucleus, which also is the base structure of chlorophyll
in plants and hemin in blood (Figure 2.27). The transformation of the
natural compounds to porphyrins probably takes place only during
sedimentation with replacement of the magnesium in chlorophyll and
iron in hemin by vanadium or nickel. This stabilizes the molecule,
insuring its preservation. Blumer and Snyder suggested that the
precursors of the porphyrins are incorporated into kerogen and are later
transformed to porphyrins during the various changes that take place as
the kerogen-type organics change to crude oil [ 171.
The alkenes are unsaturated hydrocarbons that contain double bonds
between the carbons. Thus, the balance of the carbon atoms are
not completely satisfied with hydrogen atoms. An example of this is
1-propene, in which the second and third carbon atoms are joined by
a double bond and the rest are single-bonded (Figure 2.20).
Asphalt is a black colloidal solution composed of high-molecular-weight
polynuclear aromatic compounds, high-molecular-weight unsaturated
compounds, and heterogeneous hydrocarbons containing nitrogen,
sulfur, oxygen, and metals in their structure. The heavy oils and
bitumens generally contain more nitrogen, sulfur, oxygen, and metal
compounds than do the light oils, and many oils contain free hydrogen
sulfide gas.
PROBLEMS
1. Convection currents in the mantle are apparently responsible for
the movements of continents. Explain the location (accumulation)