Page 103 - Petrophysics 2E
P. 103
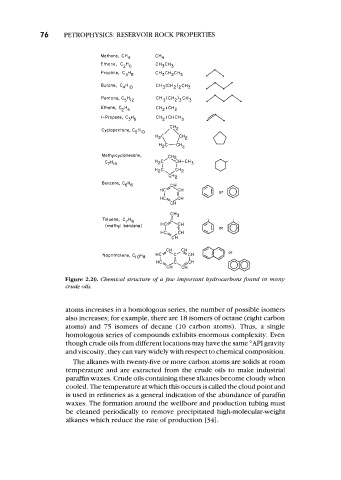
76 PETROPHYSICS: RESERVOIR ROCK PROPERTIES
Methane, CH4 CH4
Ethane, C2H6 CH3CH3
Propane, C3H8 CH~CHZCHJ A
Butane, C4HI0 CH3(CH2)2CH3 N
Pentane, C5H,2 CH3(CH213CH3 M
Ethene, C2H4 CH2:CHz
A
I-Propene, C3H6 CH2 :CHCH3
0
Cyclopentone, C5HI0
Methylcyclohexane,
C7H14
Benzene, C6H6
Figure 2.20. Chemical structure of a few important hydrocarbons found in many
crude oils.
atoms increases in a homologous series, the number of possible isomers
also increases; for example, there are 18 isomers of octane (eight carbon
atoms) and 75 isomers of decane (10 carbon atoms). Thus, a single
homologous series of compounds exhibits enormous complexity. Even
though crude oils from different locations may have the same “MI gravity
and viscosity, they can vary widely with respect to chemical composition.
The alkanes with twenty-five or more carbon atoms are solids at room
temperature and are extracted from the crude oils to make industrial
paraffin waxes. Crude oils containing these alkanes become cloudy when
cooled. The temperature at which this occurs is called the cloud point and
is used in refineries as a general indication of the abundance of paraffin
waxes. The formation around the wellbore and production tubing must
be cleaned periodically to remove precipitated high-molecular-weight
alkanes which reduce the rate of production [34].