Page 291 - Petrophysics 2E
P. 291
LAB-DERIVED EVALUATION OF SHALY 263
-CALCULATED FROM EOUATION
AVERAGE FROM CONDUCTIVITY
EXPERIMENTS, GROUP lI
I
0.02 L
UT<,
I I I I I I
0 0.0 2 0.04 0.06 0% 8
Cw , mho/cm
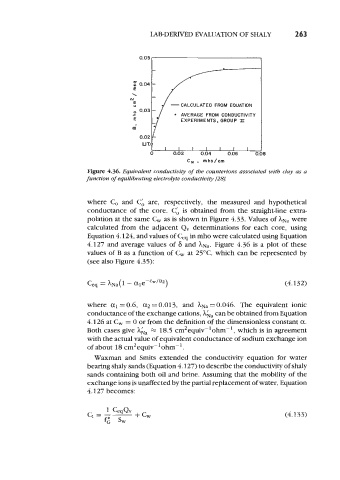
Figure 4.36. Equivalent conductivity of the counterions associated with chy as a
function of equilibrating electrolyte conductivity @8].
where C, and Cd are, respectively, the measured and hypothetical
conductance of the core. Cd is obtained from the straight-line extra-
polation at the same C, as is shown in Figure 4.33. Values of hNa were
calculated from the adjacent Qv determinations for each core, using
Equation 4.124, and values of C,, in mho were calculated using Equation
4.127 and average values of 6 and hNa. Figure 4.36 is a plot of these
values of B as a function of C, at 25OC, which can be represented by
(see also Figure 4.35):
where a1 = 0.6, a2 = 0.013, and hNa = 0.046. The equivalent ionic
conductance of the exchange cations, Aha can be obtained from Equation
4.126 at C, = 0 or from the definition of the dimensionless constant a.
Both cases give Aka M 18.5 cm2equiv-'ohm-', which is in agreement
with the actual value of equivalent conductance of sodium exchange ion
of about 18 cm2equiv-'ohm-1.
Waxman and Smits extended the conductivity equation for water
bearing shaly sands (Equation 4.127) to describe the conductivity of shaly
sands containing both oil and brine. Assuming that the mobility of the
exchange ions is unaffected by the partial replacement of water, Equation
4.127 becomes:
(4.133)