Page 91 - Photonics Essentials an introduction with experiments
P. 91
Photoconductivity
Photoconductivity 85
much less than 1%. Since photon absorption by an impurity occurs in
the physical vicinity of the impurity, the quantum efficiency of this
kind of photoconductive detector is usually much less than unity. This
may be an acceptable trade-off for the access to spectral response at a
particular photon energy, and the technological advantage of working
with a well-known host material like silicon. On the other hand, the
photoconductivity continues until an electron is trapped on the ion-
ized center. This can be a long time. Consequently, the gain, which is
given by the ratio of the time to trap an electron on the center divided
by the electron transit time, can be quite large. There are even some
kinds of devices that exhibit “persistent photoconductivity.” This
means that one exposure to light raises the conductivity of the materi-
al indefinitely, for hours or even days.
The engineering of photoconductivity is based on the intentional in-
troduction of impurity atoms or molecules in order to modify the life-
time of the photoexcited charge carriers. There are a number of varia-
tions on this theme, and we will discuss here only two of the
important applications: photographic film and sensitization.
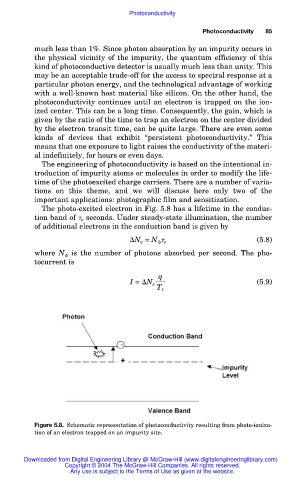
The photo-excited electron in Fig. 5.8 has a lifetime in the conduc-
tion band of e seconds. Under steady-state illumination, the number
of additional electrons in the conduction band is given by
(5.8)
N e = N e
where N is the number of photons absorbed per second. The pho-
tocurrent is
q
I = N e (5.9)
T r
Figure 5.8. Schematic representation of photoconductivity resulting from photo-ioniza-
tion of an electron trapped on an impurity site.
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2004 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.