Page 94 - Photonics Essentials an introduction with experiments
P. 94
Photoconductivity
88 Photonic Devices
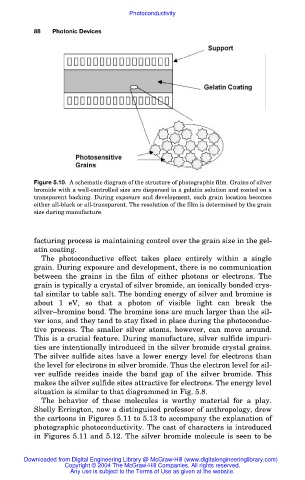
Figure 5.10. A schematic diagram of the structure of photographic film. Grains of silver
bromide with a well-controlled size are dispersed in a gelatin solution and coated on a
transparent backing. During exposure and development, each grain location becomes
either all-black or all-transparent. The resolution of the film is determined by the grain
size during manufacture.
facturing process is maintaining control over the grain size in the gel-
atin coating.
The photoconductive effect takes place entirely within a single
grain. During exposure and development, there is no communication
between the grains in the film of either photons or electrons. The
grain is typically a crystal of silver bromide, an ionically bonded crys-
tal similar to table salt. The bonding energy of silver and bromine is
about 1 eV, so that a photon of visible light can break the
silver–bromine bond. The bromine ions are much larger than the sil-
ver ions, and they tend to stay fixed in place during the photoconduc-
tive process. The smaller silver atoms, however, can move around.
This is a crucial feature. During manufacture, silver sulfide impuri-
ties are intentionally introduced in the silver bromide crystal grains.
The silver sulfide sites have a lower energy level for electrons than
the level for electrons in silver bromide. Thus the electron level for sil-
ver sulfide resides inside the band gap of the silver bromide. This
makes the silver sulfide sites attractive for electrons. The energy level
situation is similar to that diagrammed in Fig. 5.8.
The behavior of these molecules is worthy material for a play.
Shelly Errington, now a distinguised professor of anthropology, drew
the cartoons in Figures 5.11 to 5.13 to accompany the explanation of
photographic photoconductivity. The cast of characters is introduced
in Figures 5.11 and 5.12. The silver bromide molecule is seen to be
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2004 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.