Page 132 - Photoreactive Organic Thin Films
P. 132
4, PHOTOISOMERIZATION AND PHOTO-ORIENTATION OF AZO DYE IN FILMS OF POLYMER II I
(CH 2) 5
^OCzH^*"*'1*\.
OH 0 CD OH OH
SiOo Substrate
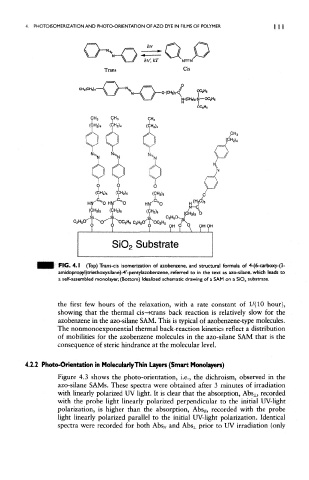
FK3. 4.1 (Top) Trans-cis isomerization of azobenzene, and structural formula of 4-(6-carboxy-(3-
amidapropyl)triethoxysilane)-4'-pentyfazobenzene, referred to in the text as azo-silane, which leads to
a self-assembled monolayer, (Bottom) Idealized schematic drawing of a SAM on a SiO x substrate.
the first few hours of the relaxation, with a rate constant of 1/(10 hour),
showing that the thermal cis-Hrans back reaction is relatively slow for the
azobenzene in the azo-silane SAM. This is typical of azobenzene-type molecules.
The nonmonoexponential thermal back-reaction kinetics reflect a distribution
of mobilities for the azobenzene molecules in the azo-silane SAM that is the
consequence of steric hindrance at the molecular level.
4.2.2 Photo-Orientation in Molecularly Thin Layers (Smart Monolayers)
Figure 4.3 shows the photo-orientation, i.e., the dichroism, observed in the
azo-silane SAMs. These spectra were obtained after 3 minutes of irradiation
with linearly polarized UV light. It is clear that the absorption, Abs 1} recorded
with the probe light linearly polarized perpendicular to the initial UV-light
polarization, is higher than the absorption, Abs//, recorded with the probe
light linearly polarized parallel to the initial UV-light polarization. Identical
spectra were recorded for both Abs// and Abs x prior to UV irradiation (only