Page 136 - Photoreactive Organic Thin Films
P. 136
4, PHOTOISOMERIZATION AND PHOTO-ORIENTATION OF AZO DYE IN FILMS OF POLYMER | | 5
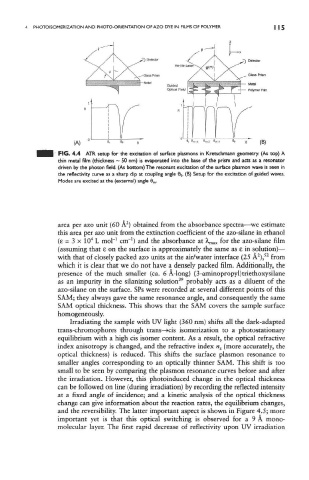
FIG. 4.4 ATR setup for the excitation of surface piasmons in Kretschmann geometry, (As top) A
thin metal film (thickness ~ 50 nm) is evaporated into the base of the prism and acts as a resonator
driven by the photon field. (As bottom) The resonant excitation of the surface plasmon wave is seen in
the reflectivity curve as a sharp dip at coupling angle 6 0. (B) Setup for the excitation of guided waves.
Modes are excited at the (external) angle 0 m.
2
area per azo unit (60 A ) obtained from the absorbance spectra—we estimate
this area per azo unit from the extinction coefficient of the azo-silane in ethanol
4
1
(E = 3 x 10 L moH cm' ) and the absorbance at X max for the azo-silane film
(assuming that e on the surface is approximately the same as e in solution)—
2 52
with that of closely packed azo units at the air/water interface (25 A ), from
which it is clear that we do not have a densely packed film. Additionally, the
presence of the much smaller (ca. 6 A-long) (3-aminopropyl)triethoxysilane
39
as an impurity in the silanizing solution probably acts as a diluent of the
azo-silane on the surface. SPs were recorded at several different points of this
SAM; they always gave the same resonance angle, and consequently the same
SAM optical thickness. This shows that the SAM covers the sample surface
homogeneously.
Irradiating the sample with UV light (360 nm) shifts all the dark-adapted
trans-chromophores through trans—>cis isomerization to a photostationary
equilibrium with a high cis isomer content. As a result, the optical refractive
index anisotropy is changed, and the refractive index n z (more accurately, the
optical thickness) is reduced. This shifts the surface plasmon resonance to
smaller angles corresponding to an optically thinner SAM. This shift is too
small to be seen by comparing the plasmon resonance curves before and after
the irradiation. However, this photoinduced change in the optical thickness
can be followed on line (during irradiation) by recording the reflected intensity
at a fixed angle of incidence; and a kinetic analysis of the optical thickness
change can give information about the reaction rates, the equilibrium changes,
and the reversibility. The latter important aspect is shown in Figure 4.5; more
important yet is that this optical switching is observed for a 9 A mono-
molecular layer. The first rapid decrease of reflectivity upon UV irradiation