Page 25 - Photoreactive Organic Thin Films
P. 25
HERMANN RAU
1. The azobenzene unit is chemically stable at moderate temperatures
and against UV/VIS radiation;
1
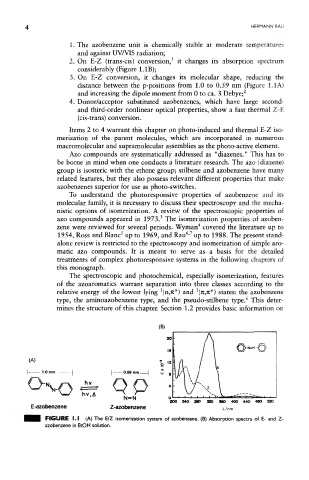
2. On E-Z (trans-cis) conversion, it changes its absorption spectrum
considerably (Figure LIB);
3. On E-Z conversion, it changes its molecular shape, reducing the
distance between the p-positions from 1.0 to 0.59 nm (Figure 1.1A)
2
and increasing the dipole moment from 0 to ca. 3 Debye;
4. Donor/acceptor substituted azobenzenes, which have large second-
and third-order nonlinear optical properties, show a fast thermal Z-E
(cis-trans) conversion.
Items 2 to 4 warrant this chapter on photo-induced and thermal E-Z iso-
merization of the parent molecules, which are incorporated in numerous
macromolecular and supramolecular assemblies as the photo-active element.
Azo compounds are systematically addressed as "diazenes." This has to
be borne in mind when one conducts a literature research. The azo (diazene)
group is isosteric with the ethene group; stilbene and azobenzene have many
related features, but they also possess relevant different properties that make
azobenzenes superior for use as photo-switches.
To understand the photoresponsive properties of azobenzene and its
molecular family, it is necessary to discuss their spectroscopy and the mecha-
nistic options of isomerization. A review of the spectroscopic properties of
3
azo compounds appeared in 1973. The isomerization properties of azoben-
4
zene were reviewed for several periods. Wyman covered the literature up to
5 6 7
1954, Ross and Blanc up to 1969, and Rau ' up to 1988. The present stand-
alone review is restricted to the spectroscopy and isomerization of simple aro-
matic azo compounds. It is meant to serve as a basis for the detailed
treatments of complex photoresponsive systems in the following chapters of
this monograph.
The spectroscopic and photochemical, especially isomerization, features
of the azoaromatics warrant separation into three classes according to the
I
l
relative energy of the lowest lying (n 9n*) and (n,n*) states: the azobenzene
6
type, the aminoazobenzene type, and the pseudo-stilbene type. This deter-
mines the structure of this chapter. Section 1.2 provides basic information on
(A)
200 240 29P 320 360 400 440 400 S»
E -azobenzene Z-azobenzene x/nm
FtCHIRE I.I (A) The E/Z isomerization system of azobenzene. (B) Absorption spectra of E- and Z-
azobenzene in EtOH solution.