Page 29 - Photoreactive Organic Thin Films
P. 29
HERMANN RAU
0.2
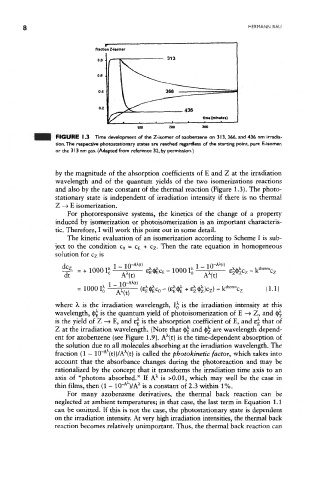
FIGURE 1.3 Time development of the Z-isomer of azobenzene on 313, 366, and 436 nm irradia-
tion. The respective photostationary states are reached regardless of the starting point, pure E-isomer,
or the 313 nm pss. (Adapted from reference 32, by permission.)
by the magnitude of the absorption coefficients of E and Z at the irradiation
wavelength and of the quantum yields of the two isomerizations reactions
and also by the rate constant of the thermal reaction (Figure 1.3). The photo-
stationary state is independent of irradiation intensity if there is no thermal
Z -» E isomerization.
For photoresponsive systems, the kinetics of the change of a property
induced by isomerization or photoisomerization is an important characteris-
tic. Therefore, I will work this point out in some detail.
The kinetic evaluation of an isomerization according to Scheme I is sub-
ject to the condition c f) = C E + c z. Then the rate equation in homogeneous
solution for c z is
10 -AMt)
= + 1000 It c^/i^/'-. _ i nnn i^ _ ,»A,AA, c
- 1000
x
t A (t) 1>z z
AX(t)
1 - 10-
= 1000 (1.1)
where X is the irradiation wavelength, ij is the irradiation intensity at this
wavelength, jfc is the quantum yield of photoisomerization of E -> Z, and <j£
is the yield of Z ~» E, and e£ is the absorption coefficient of E, and 4 that of
Z at the irradiation wavelength. (Note that <j>£ and ^ are wavelength depend-
x
ent for azobenzene (see Figure 1.9). A (t) is the time-dependent absorption of
the solution due to all molecules absorbing at the irradiation wavelength. The
fraction (1 - W^(t))/A\t) is called the photokinetic factor, which takes into
account that the absorbance changes during the photoreaction and may be
rationalized by the concept that it transforms the irradiation time axis to an
axis of "photons absorbed." If A^ is >0.01, which may well be the case in
A
thin films, then (1 - 10~ VA* is a constant of 2.3 within 1%.
For many azobenzene derivatives, the thermal back reaction can be
neglected at ambient temperatures; in that case, the last term in Equation 1.1
can be omitted. If this is not the case, the photostationary state is dependent
on the irradiation intensity. At very high irradiation intensities, the thermal back
reaction becomes relatively unimportant. Thus, the thermal back reaction can