Page 34 - Photoreactive Organic Thin Films
P. 34
i. PHOTO1SOMERIZATION OF AZOBENZENES (3
are exactly two independent reactions in the system; if not, there are more
than two (Figure 5.1, parts A through D).
« s > 2: By choosing four or more wavelengths, higher-order ADQs-diagrams
41
can be constructed with similar diagnostic value. However, the scatter
resulting from the propagation of experimental errors in dividing jeopard-
izes the usefulness of this procedure in most cases.
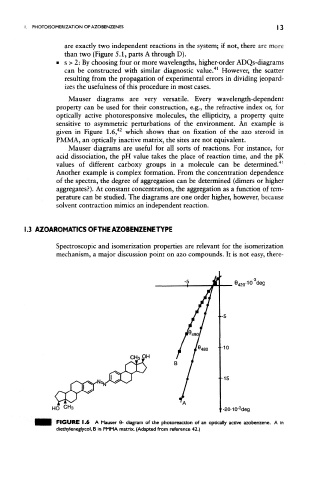
Mauser diagrams are very versatile. Every wavelength-dependent
property can be used for their construction, e.g., the refractive index or, for
optically active photoresponsive molecules, the ellipticity, a property quite
sensitive to asymmetric perturbations of the environment. An example is
42
given in Figure 1.6, which shows that on fixation of the azo steroid in
PMMA, an optically inactive matrix, the sites are not equivalent.
Mauser diagrams are useful for all sorts of reactions. For instance, for
acid dissociation, the pH value takes the place of reaction time, and the pK
45
values of different carboxy groups in a molecule can be determined.
Another example is complex formation. From the concentration dependence
of the spectra, the degree of aggregation can be determined (dimers or higher
aggregates?). At constant concentration, the aggregation as a function of tem-
perature can be studied. The diagrams are one order higher, however, because
solvent contraction mimics an independent reaction.
1.3 AZOAROMATICS OFTHE AZOBENZENETYPE
Spectroscopic and isomerization properties are relevant for the isomerization
mechanism, a major discussion point on azo compounds. It is not easy, there-
3
0 420.10" deg
-15
3
-20-10' deg
FIGURE 1.6 A Mauser 6- diagram of the photoreaction of an optically active azobenzene. A in
diethyleneglycol, B in PMMA matrix. (Adapted from reference 42.)