Page 36 - Photoreactive Organic Thin Films
P. 36
PHOTOISOMERIZATiON OF AZOBENZENES J 5
The 7i —> 7i* band in Z-azobenzene has an odd appearance. It does
not have an expressed maximum but seems to be situated at 274 nm with
1 1 43
£ = 5000 1 mol" cm"" . This indicates nonplanarity, which is also found in
16
the X-ray crystal analysis. When the structure is rigidified, such as by fixing
two ortho-positions by a -CH 2- bridge (o,o'-azo-diphenylmethane), the band
shifts to near 340 nm (indicating also more planarity) and gains intensity and
3
some structure. This indicates that on TI -» n* excitation of Z-azobenzene, a
Franck-Condon state that is on a slope of the potential energy surface of the
}
(n,K*) state is reached.
1.3.1.1.3 Influence of Substitution
Many substituted azobenzenes belong to the azobenzene type, as well.
Substitution may shift the n —» n* band from 440 nm (azobenzene), to
465 nm (hexamethylazobenzene), to 480 nm (2,2'-dimethyl-4,6,4',6'~tetra-
tert.butylazobenzene), and even to 520 nm (hexaphenylazobenzene).
48
Hydrocarbon, halogen, nitro, carboxy, acetyl, hydroxy, m-amino and even
49
2,2'4,4',6,6' hexaphenyl substitution influences the (7t,7i*)-(n,7C*) state ener-
gy gap, but not so much as to shift the molecule into the aminoazobenzene
group. In the context of this book, it is important that the long-chain and
polymeric molecules containing azobenzene units coupled by means of
hydroxy and carboxy substituents are of the azobenzene type.
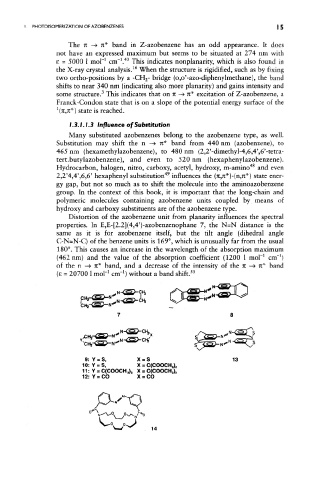
Distortion of the azobenzene unit from planarity influences the spectral
properties. In E,E-[2.2](4,4')-azobenzenophane 7, the N=N distance is the
same as it is for azobenzene itself, but the tilt angle (dihedral angle
C-N=N-C) of the benzene units is 169°, which is unusually far from the usual
180°. This causes an increase in the wavelength of the absorption maximum
1
(462 nm) and the value of the absorption coefficient (1200 1 moH cm" )
of the n —» TC* band, and a decrease of the intensity of the 71 —> it* band
1 1 50
£ = 20700 1 mol' cm" without a band shift.
9:Y = S, X = S 13
10:Y = S, X = C(COOCH 3 ) 2
11: Y = C(COOCH 3) 2 X = C(COOCH 3 ) 2
12:Y = CO X = CO
14