Page 37 - Photoreactive Organic Thin Films
P. 37
HERMANN RAU
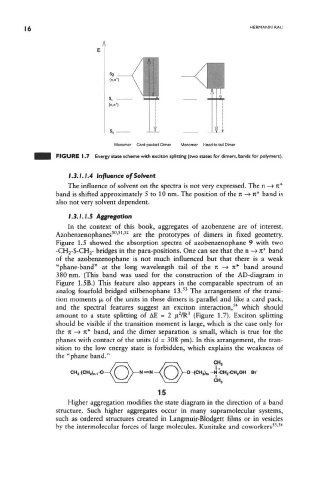
FIGURE 1.7 Energy state scheme with exciton splitting (two states for dimers, bands for polymers).
1.3. LI.4 Influence of Solvent
The influence of solvent on the spectra is not very expressed. The n —> n*
band is shifted approximately 5 to 10 nm. The position of the n —» n* band is
also not very solvent dependent.
1.3.1.1.5 Aggregation
In the context of this book, aggregates of azobenzene are of interest.
50 51 52
Azobenzenophanes ' ' are the prototypes of dimers in fixed geometry.
Figure 1.5 showed the absorption spectra of azobenzenophane 9 with two
-CH 2-S-CH 2- bridges in the para-positions. One can see that the n —> xc* band
of the azobenzenophane is not much influenced but that there is a weak
"phane-band" at the long wavelength tail of the jc —» TC* band around
380 nm. (This band was used for the construction of the AD-diagrarn in
Figure 1.5B.) This feature also appears in the comparable spectrum of an
53
analog fourfold bridged stilbenophane 13. The arrangement of the transi-
tion moments u, of the units in these dimers is parallel and like a card pack,
54
and the spectral features suggest an exciton interaction, which should
2
3
amount to a state splitting of AE = 2 fj, /R (Figure 1.7). Exciton splitting
should be visible if the transition moment is large, which is the case only for
the n —> it* band, and the dimer separation is small, which is true for the
phanes with contact of the units (d = 308 pm). In this arrangement, the tran-
sition to the low energy state is forbidden, which explains the weakness of
the "phane band."
CH 3-(CH 2) n. rO-U ))—N— N—(( )V-0-(CH 2) m-N<;H 2.CH 2OH Br
15
Higher aggregation modifies the state diagram in the direction of a band
structure. Such higher aggregates occur in many supramolecular systems,
such as ordered structures created in Langmuir-Blodgett films or in vesicles
55 56
by the intermolecular forces of large molecules. Kunitake and coworkers '