Page 38 - Photoreactive Organic Thin Films
P. 38
PHOTOISOMERIZATION OF AZOBENZENES 17
lead a very detailed investigation on bilayer assemblies of different amphi-
55
phiies of the type of 15. They have used the effect of temperature on aggre-
gation and bring their spectral results, which are obtained for different
azobenzene amphiphiles of the p,p'-dihydroxy series, in line. The maximum
of the monomeric unit is around 355 nm; card-packed aggregates absorb at
lower wavelengths, down to 300 nm; head-to-tail arrangements, as verified in
the tilted bilayers, lead to high-intensity bands at longer wavelengths up to
400 nm. The influence of packing also becomes evident in the structure that
appears on the exciton spectra of the head-to-tail aggregates. 56
I.3JJ.6 Emission
Azobenzene-type azoaromatics generally do not emit with reasonable
quantum yields after excitation either to the ^n,?**) or to the ^rc*) states.
There are only very few reports, and in most of them powerful lasers that
7
allow detection of quantum yields down to 5-10~ are used. Struve reported a
short-lived fluorescence of azobenzene in cyclohexane from the (n,rc*) state
58
57
(T = 25 ps) and even from the second excited (jc,7t*) state (t = 5 ps), a find-
ing that violates Kasha's rule, but gains support from the fact of the large
l
'(n,ii*) - (nji*) state separation. In a conference contribution, Hamai and
59
Hirayama have presented confirmative results and given a (n,Ji;*) -» S 0
5
quantum yield of 1.7-10" and a calculated lifetime of 0.06 ps. Azuma et al. &0
report that the liquid crystalline £raws-4-butyl-4'-methoxyazobenzene diluted
in hexane, which according to its absorption spectrum clearly is of the
l
azobenzene type, emits from the (n tn*) state (S 2) at 400 nm with about
0.25 ps. Although the residuals are not too good, the result is supported by
l
femtosecond absorption measurements which show that the (n,n*} state (S 5 )
is populated with the same time constant. This state lives about 2.3 ps,
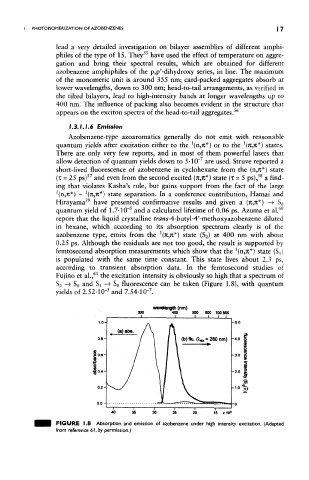
according to transient absorption data. In the femtosecond studies of
61
Fujino et al., the excitation intensity is obviously so high that a spectrum of
S 2 -> S 0 and Sj —» S 0 fluorescence can be taken (Figure 1.8), with quantum
7
5
yields of 2.52-KT and 7.54-1Q- .
wavelength (nm)
300 400 500 600 700 800
J I I I I I
!0.6-
!0.4-
15 xlO 3
FIGURE 1.8 Absorption and emission of azobenzene under high intensity excitation. (Adapted
from reference 61, by permission.)