Page 43 - Photoreactive Organic Thin Films
P. 43
22 HERMANN RAU
0,0
200 250 300 350 400 450 5 550 600
wavelength / nm
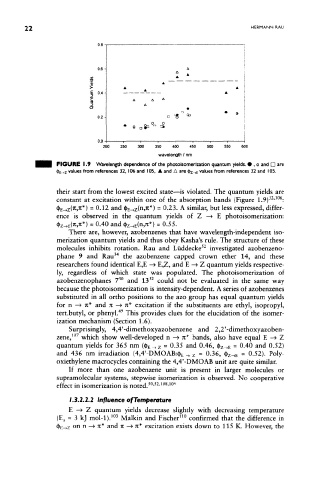
FIGURE 1 .9 Wavelength dependence of the photoisomerization quantum yields. • , o and D are
<|>E_»Z values from references 32, 106 and 105, A and A are <|>Z-»E values from references 32 and 105,
their start from the lowest excited state—is violated. The quantum yields are
32 106
constant at excitation within one of the absorption bands (Figure 1.9) ' :
n w
fyE-tzfaJi*) - 0-12 and $E-»z( » *) - 0.23. A similar, but less expressed, differ-
ence is observed in the quantum yields of Z —> E photoisomerization:
<&£-*(«,**) = 0-40 and <h^ E(n,JC*) = 0.55.
There are, however, azobenzenes that have wavelength-independent iso-
merization quantum yields and thus obey Kasha's rule. The structure of these
52
molecules inhibits rotation. Rau and Luddecke investigated azobenzeno-
34
phane 9 and Rau the azobenzene capped crown ether 14, and these
researchers found identical E,E -~» E,Z, and E —» Z quantum yields respective-
ly, regardless of which state was populated. The photoisomerization of
50 52
azobenzenophanes 7 and 13 could not be evaluated in the same way
because the photoisomerization is intensity-dependent. A series of azobenzenes
substituted in all ortho positions to the azo group has equal quantum yields
for n —> n* and K —» it* excitation if the substituents are ethyl, isopropyl,
49
tert.butyl, or phenyl. This provides clues for the elucidation of the isomer-
ization mechanism (Section 1.6).
Surprisingly, 4,4'-dimethoxyazobenzene and 2,2'-dimethoxyazoben-
107
zene, which show well-developed n —> TC* bands, also have equal E —» Z
quantum yields for 365 nm (<j> E _» z = 0.35 and 0.46, <|>Z-»E = 0.40 and 0.52)
and 436 nm irradiation (4,4'-DMOAB:<|> E _» z - 0.36, <|> Z^ E = 0.52). Poly-
oxiethylene macrocycles containing the 4,4'-DMOAB unit are quite similar.
If more than one azobenzene unit is present in larger molecules or
supramolecular systems, stepwise isomerization is observed. No cooperative
50 52 108 109
effect in isomerization is noted. ' ' '
/.3.2.2.2 Influence of Temperature
E —> Z quantum yields decrease slightly with decreasing temperature
110
103
(E a ~ 3 kj mol-1). Malkin and Fischer confirmed that the difference in
%i~»z on n -> 7t* and K —> n* excitation exists down to 115 K. However, the