Page 47 - Photoreactive Organic Thin Films
P. 47
26 HERMANN RAD
350 300 260
1.6
1.2
0.8
0.4
0
300 350 400 450 600
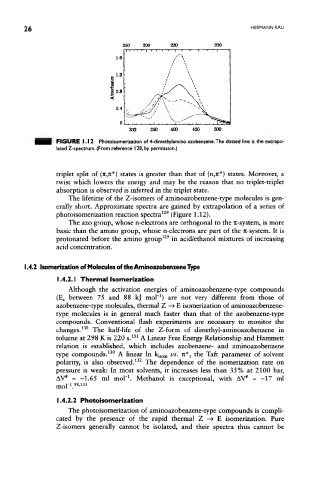
FKHflUE 1.12 Photoisomerization of 4-dimethylamino azobenzene.The dotted line is the extrapo-
lated Z-spectrum. (From reference 128, by permission.)
triplet split of (n,K*) states is greater than that of (n,7t*) states. Moreover, a
twist which lowers the energy and may be the reason that no triplet-triplet
absorption is observed is inferred in the triplet state.
The lifetime of the Z-isomers of aminoazobenzene-type molecules is gen-
erally short. Approximate spectra are gained by extrapolation of a series of
128
photoisomerization reaction spectra (Figure 1.12).
The azo group, whose n-electrons are orthogonal to the 7c-system, is more
basic than the amino group, whose n-electrons are part of the TC-system. It is
129
protonated before the amino group in acid/ethanol mixtures of increasing
acid concentration.
1.4.2 Isomerization of Molecules of the Aminoazobenzene Type
1.4.2.1 Thermal Isomerization
Although the activation energies of aminoazobenzene-type compounds
(E a between 75 and 88 kj moH) are not very different from those of
azobenzene-type molecules, thermal Z —> E isomerization of aminoazobenzene-
type molecules is in general much faster than that of the azobenzene-type
compounds. Conventional flash experiments are necessary to monitor the
130
changes. The half-life of the Z-form of dimethyl-aminoazobenzene in
131
toluene at 298 K is 220 s. A Linear Free Energy Relationship and Hammett
relation is established, which includes azobenzene- and aminoazobenzene
130
type compounds. A linear In k isom vs. ft*, the Taft parameter of solvent
132
polarity, is also observed. The dependence of the isomerization rate on
pressure is weak: In most solvents, it increases less than 35% at 2100 bar,
AV* « -1.65 ml moH. Methanol is exceptional, with AV* = -17 ml
1 88 133
mol- . '
1.4.2.2 Photoisomerization
The photoisomerization of aminoazobenzene-type compounds is compli-
cated by the presence of the rapid thermal Z —» E isomerization. Pure
Z-isomers generally cannot be isolated, and their spectra thus cannot be