Page 44 - Photoreactive Organic Thin Films
P. 44
PHOTOISOMERiZATION OFAZOBENZENES 23
(436 nm)
(313 nm)
•e-
c
<h=_ z (313 ran)
0.004 0006 0,008 0.010 0.015
1/T/tC 1
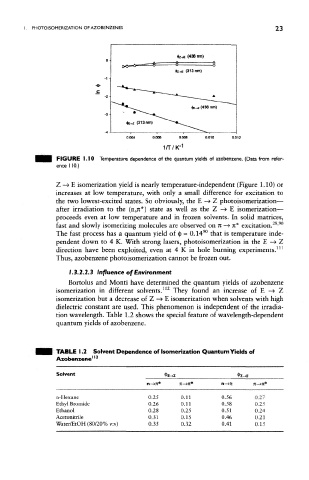
FIGURE 1. 10 Temperature dependence of the quantum yields of azobenzene. (Data from refer-
ence 110.)
Z —» E isomerization yield is nearly temperature-independent (Figure 1,10) or
increases at low temperature, with only a small difference for excitation to
the two lowest-excited states. So obviously, the E —> Z photoisomerization—
after irradiation to the (n,rc*) state as well as the Z —> E isomerization—
proceeds even at low temperature and in frozen solvents. In solid matrices,
28 90
fast and slowly isomerizing molecules are observed on n -» n* excitation. '
90
The fast process has a quantum yield of § ~ 0.14 that is temperature inde-
pendent down to 4 K. With strong lasers, photoisomerization in the E —» Z
111
direction have been exploited, even at 4 K in hole burning experiments.
Thus, azobenzene photoisomerization cannot be frozen out.
f.3.2.2.3 Influence of Environment
Bortolus and Monti have determined the quantum yields of azobenzene
112
isomerization in different solvents. They found an increase of E -> Z
isomerization but a decrease of Z —> E isomerization when solvents with high
dielectric constant are used. This phenomenon is independent of the irradia-
tion wavelength. Table 1.2 shows the special feature of wavelength-dependent
quantum yields of azobenzene.
TABLE 1.2 Solvent Dependence of Isomerization Quantum Yields of
Azobenzene" 2
Solvent (bg^z <J>Z-»E
n—>TI*
n-Hexane 0.25 0.11 0.56 0.27
Ethyl Bromide 0.26 0.11 0.58 0.25
Ethanol 0.28 0.25 0.51 0.24
Acetonitrile 0.31 0.15 0.46 0.21
Water/EtOH (80/20% v:v) 0.35 0.32 0.41 0.15