Page 49 - Photoreactive Organic Thin Films
P. 49
28 HERMANN RAU
- -,- 120
400 500
/nm
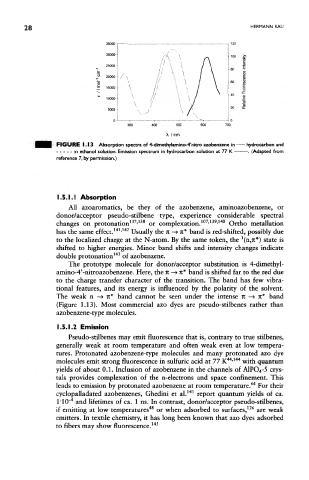
FIGURE 1.13 Absorption spectra of 4-dimethylamino-4'nitro azobenzene in - hydrocarbon and
in ethanol solution. Emission spectrum in hydrocarbon solution at 77 K - —. (Adapted from
reference 7, by permission.)
1.5.1.1 Absorption
All azoaromatics, be they of the azobenzene, aminoazobenzene, or
donor/acceptor pseudo-stilbene type, experience considerable spectral
137 138 107 139 140
changes on protonation ' or complexation. ' ' Ortho metallation
141 142
has the same effect. ' Usually the n -> n* band is red-shifted, possibly due
1
to the localized charge at the N-atom. By the same token, the (n,n*) state is
shifted to higher energies. Minor band shifts and intensity changes indicate
143
double protonation of azobenzene.
The prototype molecule for donor/acceptor substitution is 4-dimethyl-
aminO"4'-nitroazobenzene. Here, the n —> K* band is shifted far to the red due
to the charge transfer character of the transition. The band has few vibra-
tional features, and its energy is influenced by the polarity of the solvent.
The weak n —> it* band cannot be seen under the intense n —» ft* band
(Figure 1.13). Most commercial azo dyes are pseudo-stilbenes rather than
azobenzene-type molecules.
1.5.1.2 Emission
Pseudo-stilbenes may emit fluorescence that is, contrary to true stilbenes,
generally weak at room temperature and often weak even at low tempera-
tures. Protonated azobenzene-type molecules and many protonated azo dye
44 144
molecules emit strong fluorescence in sulfuric acid at 77 K ' with quantum
yields of about 0.1. Inclusion of azobenzene in the channels of AlPO 4-5 crys-
tals provides complexation of the n-eleetrons and space confinement. This
64
leads to emission by protonated azobenzene at room temperature. For their
141
cyclopalladated azobenzenes, Ghedini et al. report quantum yields of ca.
4
110" and lifetimes of ca. 1 ns. In contrast, donor/acceptor pseudo-stilbenes,
126
48
if emitting at low temperatures or when adsorbed to surfaces, are weak
emitters. In textile chemistry, it has long been known that azo dyes adsorbed
to fibers may show fluorescence. 145