Page 53 - Photoreactive Organic Thin Films
P. 53
32 HERMANN RAU
inversion
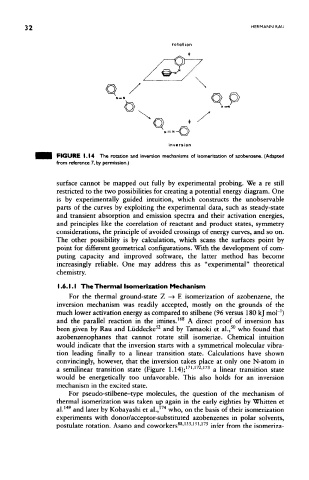
FIGURE 1.14 The rotation and inversion mechanisms of isomerization of azobenzene, (Adapted
from reference 7, by permission.)
surface cannot be mapped out fully by experimental probing. We a re still
restricted to the two possibilities for creating a potential energy diagram. One
is by experimentally guided intuition, which constructs the unobservable
parts of the curves by exploiting the experimental data, such as steady-state
and transient absorption and emission spectra and their activation energies,
and principles like the correlation of reactant and product states, symmetry
considerations, the principle of avoided crossings of energy curves, and so on.
The other possibility is by calculation, which scans the surfaces point by
point for different geometrical configurations. With the development of com-
puting capacity and improved software, the latter method has become
increasingly reliable. One may address this as "experimental" theoretical
chemistry.
1.6.1.1 The Thermal Isomerization Mechanism
For the thermal ground-state Z —» E isomerization of azobenzene, the
inversion mechanism was readily accepted, mostly on the grounds of the
much lower activation energy as compared to stilbene (96 versus 180 kj moH)
168
and the parallel reaction in the imines. A direct proof of inversion has
52
50
been given by Rau and Liiddecke and by Tamaoki et al., who found that
azobenzenophanes that cannot rotate still isomerize. Chemical intuition
would indicate that the inversion starts with a symmetrical molecular vibra-
tion leading finally to a linear transition state. Calculations have shown
convincingly, however, that the inversion takes place at only one N-atom in
171 172 173
a semilinear transition state (Figure 1.14); ' ' a linear transition state
would be energetically too unfavorable. This also holds for an inversion
mechanism in the excited state.
For pseudo-stilbene-type molecules, the question of the mechanism of
thermal isomerization was taken up again in the early eighties by Whitten et
149 174
al. and later by Kobayashi et al., who, on the basis of their isomerization
experiments with donor/acceptor-substituted azobenzenes in polar solvents,
88 133 151 175
postulate rotation. Asano and coworkers ' ' ' infer from the isorneriza-