Page 56 - Photoreactive Organic Thin Films
P. 56
PHOTO1SOMER1ZATION OF AZOBENZENES 35
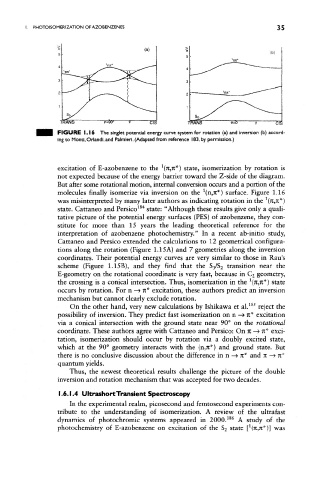
TRANS <P=90° CIS
FIGURE 1. 16 The singlet potential energy curve system for rotation (a) and inversion (b) accord-
ing to Monti, Orlandi, and Palmieri. (Adapted from reference 183, by permission.)
l
excitation of E-azobenzene to the (n,n*) state, isomerization by rotation is
not expected because of the energy barrier toward the Z-side of the diagram.
But after some rotational motion, internal conversion occurs and a portion of the
l
molecules finally isomerize via inversion on the (n,n*) surface. Figure 1.16
l
was misinterpreted by many later authors as indicating rotation in the (n,n*)
184
state. Cattaneo and Persico state: "Although these results give only a quali-
tative picture of the potential energy surfaces (PES) of azobenzene, they con-
stitute for more than 15 years the leading theoretical reference for the
interpretation of azobenzene photochemistry." In a recent ab-initio study,
Cattaneo and Persico extended the calculations to 12 geometrical configura-
tions along the rotation (Figure LISA) and 7 geometries along the inversion
coordinates. Their potential energy curves are very similar to those in Rau's
scheme (Figure 1.15B), and they find that the S 3/S 2 transition near the
E-geometry on the rotational coordinate is very fast, because in C 2 geometry,
l
the crossing is a conical intersection. Thus, isomerization in the (n,n*) state
occurs by rotation. For n —»7t* excitation, these authors predict an inversion
mechanism but cannot clearly exclude rotation.
ifc>
On the other hand, very new calculations by Ishikawa et al. reject the
possibility of inversion. They predict fast isomerization on n —> ft* excitation
via a conical intersection with the ground state near 90° on the rotational
coordinate. These authors agree with Cattaneo and Persico: On n -» n* exci-
tation, isomerization should occur by rotation via a doubly excited state,
which at the 90° geometry interacts with the (n,7i*) and ground state. But
there is no conclusive discussion about the difference in n —> K* and n —» n*
quantum yields.
Thus, the newest theoretical results challenge the picture of the double
inversion and rotation mechanism that was accepted for two decades.
1.6.1.4 Ultrashort Transient Spectroscopy
In the experimental realm, picosecond and femtosecond experiments con-
tribute to the understanding of isomerization. A review of the ultrafast
186
dynamics of photochromic systems appeared in 2000. A study of the
l
photochemistry of E-azobenzene on excitation of the S 2 state [ (n,n*)} was