Page 46 - Photoreactive Organic Thin Films
P. 46
PHOTOISOMERIZATiON OFAZOBENZENES 25
X /nm
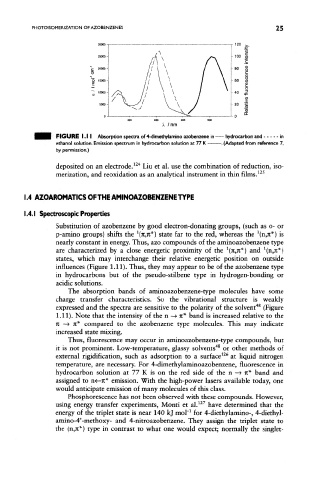
FIGURE 1. 11 Absorption spectra of 4-dimethylamino azobenzene in hydrocarbon and in
ethanol solution. Emission spectrum in hydrocarbon solution at 77 K . (Adapted from reference 7,
by permission.)
124
deposited on an electrode. Liu et al. use the combination of reduction, iso-
merization, and reoxidation as an analytical instrument in thin films. 125
1.4 AZOARONATICS OFTHE AMINOAZOBENZENETYPE
1.4.1 Spectroscopic Properties
Substitution of azobenzene by good electron-donating groups, (such as o- or
l
1
p-amino groups) shifts the (n,n*} state far to the red, whereas the (n,n*} is
nearly constant in energy. Thus, azo compounds of the aminoazobenzene type
l
1
are characterized by a close energetic proximity of the (n,n*) and (n,jc*)
states, which may interchange their relative energetic position on outside
influences (Figure 1.11). Thus, they may appear to be of the azobenzene type
in hydrocarbons but of the pseudo-stilbene type in hydrogen-bonding or
acidic solutions.
The absorption bands of aminoazobenzene-type molecules have some
charge transfer characteristics. So the vibrational structure is weakly
48
expressed and the spectra are sensitive to the polarity of the solvent (Figure
1.11). Note that the intensity of the n —» n* band is increased relative to the
n —> n* compared to the azobenzene type molecules. This may indicate
increased state mixing.
Thus, fluorescence may occur in aminoazobenzene-type compounds, but
48
it is not prominent. Low-temperature, glassy solvents or other methods of
126
external rigidification, such as adsorption to a surface at liquid nitrogen
temperature, are necessary. For 4-dimethylaminoazobenzene, fluorescence in
hydrocarbon solution at 77 K is on the red side of the n —> n* band and
assigned to n«—n* emission. With the high-power lasers available today, one
would anticipate emission of many molecules of this class.
Phosphorescence has not been observed with these compounds. However,
127
using energy transfer experiments, Monti et al. have determined that the
energy of the triplet state is near 140 kj moH for 4-diethylamino-, 4-diethyl-
amino-4'-methoxy- and 4-nitroazobenzene. They assign the triplet state to
the (n,7i*) type in contrast to what one would expect; normally the singlet-