Page 27 - Photoreactive Organic Thin Films
P. 27
HERMANN RAU
13
Although there are some "reluctant" aliphatic azo compounds, in
14
general these molecules are photochemically not very stable. Thus, they are
not used in the systems covered in this book and will not be reviewed in this
contribution.
2 15
In aromatic azo compounds, the n system is extended. X-ray data ' for
E-azobenzene give the N=N distance as 124.7 pm, not much different from
that in azomethane, and the C-N distance as 142.8 pm. The NNC angle is
2
114.1°, somewhat off the sp hybridization angle, and the molecule is planar
(>CNNC = 180°). The corresponding values for Z-azobenzene are: N=N
125.3 pm, C-N 144.9 pm, >NNC 121.9°, >CNNC 172°, and the twist angle
16
of the phenyl rings is 53.3°. This is in agreement with earlier work. Electron
17
diffraction data for E-azobenzene do not differ more than 2 pm from the
X-ray results, but they indicate a small twist angle >C-N of 30°.
The extension of the conjugation system increases the photostability of
the molecules and lowers the excitation energies compared to those of the
aliphatic compounds: The n —> n* absorption of aromatic azo compounds
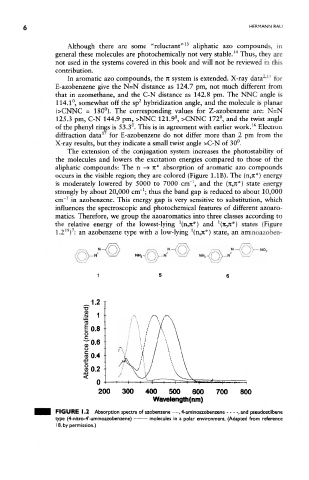
occurs in the visible region; they are colored (Figure LIB). The (n,ic*) energy
1
is moderately lowered by 5000 to 7000 cm" , and the (71,71*) state energy
1
strongly by about 20,000 cm" ; thus the band gap is reduced to about 10,000
1
cm" in azobenzene. This energy gap is very sensitive to substitution, which
influences the spectroscopic and photochemical features of different azoaro-
matics. Therefore, we group the azoaromatics into three classes according to
l
l
the relative energy of the lowest-lying (n,7t*} and (K in*) states (Figure
l
18 7
1.2 ) : an azobenzene type with a low-lying (n,Jt*) state, an aminoazoben-
200 300 400 500 600 700 800
FIGURE 1.2 Absorption spectra of azobenzene , 4-aminoazobenzene , and pseudostilbene
type (4-nitro-4'-aminoazobenzene) molecules in a polar environment. (Adapted from reference
18, by permission.)