Page 130 - Physical Principles of Sedimentary Basin Analysis
P. 130
112 Heat flow
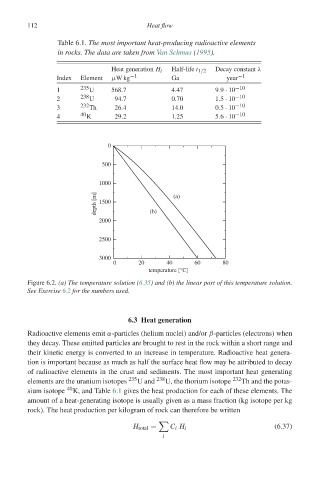
Table 6.1. The most important heat-producing radioactive elements
in rocks. The data are taken from Van Schmus (1995).
Heat generation H i Half-life t 1/2 Decay constant λ
Index Element μWkg −1 Ga year −1
1 235 U 568.7 4.47 9.9 · 10 −10
2 238 U 94.7 0.70 1.5 · 10 −10
3 232 Th 26.4 14.0 0.5 · 10 −10
4 40 K 29.2 1.25 5.6 · 10 −10
0
500
1000
depth [m] 1500 (b) (a)
2000
2500
3000
0 20 40 60 80
temperature [°C]
Figure 6.2. (a) The temperature solution (6.35) and (b) the linear part of this temperature solution.
See Exercise 6.2 for the numbers used.
6.3 Heat generation
Radioactive elements emit α-particles (helium nuclei) and/or β-particles (electrons) when
they decay. These emitted particles are brought to rest in the rock within a short range and
their kinetic energy is converted to an increase in temperature. Radioactive heat genera-
tion is important because as much as half the surface heat flow may be attributed to decay
of radioactive elements in the crust and sediments. The most important heat generating
elements are the uranium isotopes 235 U and 238 U, the thorium isotope 232 Th and the potas-
sium isotope 40 K, and Table 6.1 gives the heat production for each of these elements. The
amount of a heat-generating isotope is usually given as a mass fraction (kg isotope per kg
rock). The heat production per kilogram of rock can therefore be written
H total = C i H i (6.37)
i