Page 165 - Physical chemistry understanding our chemical world
P. 165
132 REACTION SPONTANEITY AND THE DIRECTION OF THERMODYNAMIC CHANGE
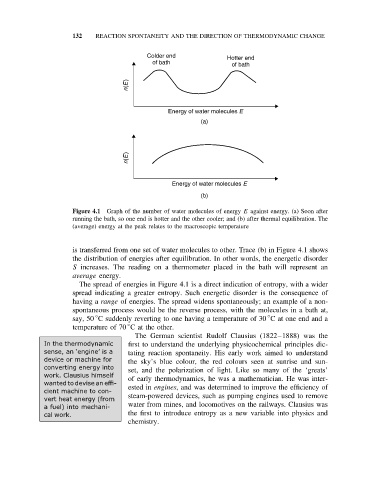
Colder end Hotter end
of bath of bath
n(E)
Energy of water molecules E
(a)
n(E)
Energy of water molecules E
(b)
Figure 4.1 Graph of the number of water molecules of energy E against energy. (a) Soon after
running the bath, so one end is hotter and the other cooler; and (b) after thermal equilibration. The
(average) energy at the peak relates to the macroscopic temperature
is transferred from one set of water molecules to other. Trace (b) in Figure 4.1 shows
the distribution of energies after equilibration. In other words, the energetic disorder
S increases. The reading on a thermometer placed in the bath will represent an
average energy.
The spread of energies in Figure 4.1 is a direct indication of entropy, with a wider
spread indicating a greater entropy. Such energetic disorder is the consequence of
having a range of energies. The spread widens spontaneously; an example of a non-
spontaneous process would be the reverse process, with the molecules in a bath at,
◦
◦
say, 50 C suddenly reverting to one having a temperature of 30 C at one end and a
◦
temperature of 70 C at the other.
The German scientist Rudolf Clausius (1822–1888) was the
In the thermodynamic first to understand the underlying physicochemical principles dic-
sense, an ‘engine’ is a tating reaction spontaneity. His early work aimed to understand
device or machine for the sky’s blue colour, the red colours seen at sunrise and sun-
converting energy into
set, and the polarization of light. Like so many of the ‘greats’
work. Clausius himself of early thermodynamics, he was a mathematician. He was inter-
wanted to devise an effi-
cient machine to con- ested in engines, and was determined to improve the efficiency of
vert heat energy (from steam-powered devices, such as pumping engines used to remove
a fuel) into mechani- water from mines, and locomotives on the railways. Clausius was
cal work. the first to introduce entropy as a new variable into physics and
chemistry.