Page 170 - Physical chemistry understanding our chemical world
P. 170
THE DIRECTION OF PHYSICOCHEMICAL CHANGE: ENTROPY 137
Why does crystallization of a solute occur?
Thermodynamic systems and universes
Atoms or ions of solute leave solution during the process of crystallization to form a
regular repeat lattice.
The extent of solute disorder is high before crystallization, be-
cause each ion or molecule resides in solution, and thereby expe-
The extent of solute
riences the same freedom as a molecule of liquid. Conversely,
disorder decreases dur-
the extent of disorder after crystallization will inevitably be much
ing crystallization.
smaller, since solute is incorporated within a solid comprising a
regular repeat lattice.
The value of S can only be negative because the symbol ‘ ’ means ‘final state
minus initial state’, and the extent of disorder during crystallization clearly follows the
order ‘solute disorder (initial) > solute disorder (final) ’. We see how the extent of disorder
in the solute decreases during crystallization in consequence of forming a lattice and,
therefore, do not expect crystallization to be a spontaneous process.
But crystallization does occur, causing us to ask, ‘Why does
crystallization occur even though S for the process is negative?’
A process is thermody-
To answer this question, we must consider all energetic consid-
namically spontaneous
erations occurring during the process of crystallization, possibly
only if the ‘overall’ value
including phenomena not directly related to the actual processes
of S is positive.
inside the beaker.
Before crystallization, each particle of solute is solvated. As
a simple example, a chloride ion in water is attached to six water molecules, as
−
[Cl(H 2 O) 6 ] . Being bound to a solute species limits the freedom of solvent molecules,
that is, when compared with free, unbound solvent.
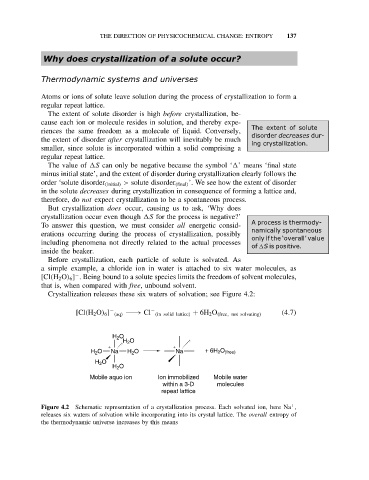
Crystallization releases these six waters of solvation; see Figure 4.2:
− −
[Cl(H 2 O) 6 ] (aq) −−→ Cl (in solid lattice) + 6H 2 O (free, not solvating) (4.7)
H 2 O
H 2 O
+ +
H 2 O Na H 2 O Na + 6H 2 O (free)
H 2 O
H 2 O
Mobile aquo ion Ion immobilized Mobile water
within a 3-D molecules
repeat lattice
+
Figure 4.2 Schematic representation of a crystallization process. Each solvated ion, here Na ,
releases six waters of solvation while incorporating into its crystal lattice. The overall entropy of
the thermodynamic universe increases by this means