Page 166 - Physical chemistry understanding our chemical world
P. 166
THE DIRECTION OF PHYSICOCHEMICAL CHANGE: ENTROPY 133
Why does a room containing oranges acquire
their aroma?
Spontaneity and the sign of S
When a bowl containing fresh oranges is placed on the dining room
We sometimes say
table, the room acquires their fragrance within a few hours. The
these compounds
organic substance we smell after its release from the oranges is the volatilize.
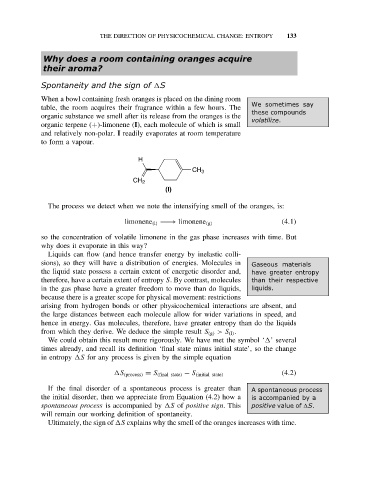
organic terpene (+)-limonene (I), each molecule of which is small
and relatively non-polar. I readily evaporates at room temperature
to form a vapour.
H
CH 3
CH 2
(I)
The process we detect when we note the intensifying smell of the oranges, is:
limonene (l) −−→ limonene (g) (4.1)
so the concentration of volatile limonene in the gas phase increases with time. But
why does it evaporate in this way?
Liquids can flow (and hence transfer energy by inelastic colli-
sions), so they will have a distribution of energies. Molecules in Gaseous materials
the liquid state possess a certain extent of energetic disorder and, have greater entropy
therefore, have a certain extent of entropy S. By contrast, molecules than their respective
in the gas phase have a greater freedom to move than do liquids, liquids.
because there is a greater scope for physical movement: restrictions
arising from hydrogen bonds or other physicochemical interactions are absent, and
the large distances between each molecule allow for wider variations in speed, and
hence in energy. Gas molecules, therefore, have greater entropy than do the liquids
from which they derive. We deduce the simple result S (g) >S (l) .
We could obtain this result more rigorously. We have met the symbol ‘ ’ several
times already, and recall its definition ‘final state minus initial state’, so the change
in entropy S for any process is given by the simple equation
(4.2)
S (process) = S (final state) − S (initial state)
If the final disorder of a spontaneous process is greater than A spontaneous process
the initial disorder, then we appreciate from Equation (4.2) how a is accompanied by a
spontaneous process is accompanied by S of positive sign.This positive value of S.
will remain our working definition of spontaneity.
Ultimately, the sign of S explains why the smell of the oranges increases with time.