Page 173 - Physical chemistry understanding our chemical world
P. 173
140 REACTION SPONTANEITY AND THE DIRECTION OF THERMODYNAMIC CHANGE
Sections 3.1 and 3.2 describe heat capacity and explain how it
The heat capacity
C p(ice) = 39 J K −1 mol −1 may be determined at constant pressure C p or at constant volume
tells us that adding 39 J C V . Most chemists need to make calculations with C p , which repre-
of energy increases the sents the amount of energy (in the form of heat) that can be stored
temperature of 1 mol within a substance – the measurement having been performed at
of water by 1 K. constant pressure p. For example, the heat capacity of solid water
−1
(ice) is 39 J K −1 mol . The value of C p for liquid water is higher,
−1
at 75 J K −1 mol , so we store more energy in liquid water than when it is solid;
stated another way, we need to add more energy to H 2 O (l) if its temperature is to
−1
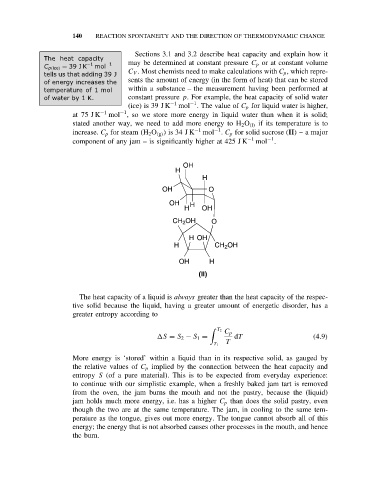
increase. C p for steam (H 2 O (g) ) is 34 J K −1 mol . C p for solid sucrose (II) – a major
−1
component of any jam – is significantly higher at 425 J K −1 mol .
OH
H
H
OH O
OH H
H OH
CH 2 OH O
H OH
H CH 2 OH
OH H
(II)
The heat capacity of a liquid is always greater than the heat capacity of the respec-
tive solid because the liquid, having a greater amount of energetic disorder, has a
greater entropy according to
T 2
C p
S = S 2 − S 1 = dT (4.9)
T
T 1
More energy is ‘stored’ within a liquid than in its respective solid, as gauged by
the relative values of C p implied by the connection between the heat capacity and
entropy S (of a pure material). This is to be expected from everyday experience:
to continue with our simplistic example, when a freshly baked jam tart is removed
from the oven, the jam burns the mouth and not the pastry, because the (liquid)
jam holds much more energy, i.e. has a higher C p than does the solid pastry, even
though the two are at the same temperature. The jam, in cooling to the same tem-
perature as the tongue, gives out more energy. The tongue cannot absorb all of this
energy; the energy that is not absorbed causes other processes in the mouth, and hence
the burn.