Page 198 - Physical chemistry understanding our chemical world
P. 198
THERMODYNAMICS AND THE EXTENT OF REACTION 165
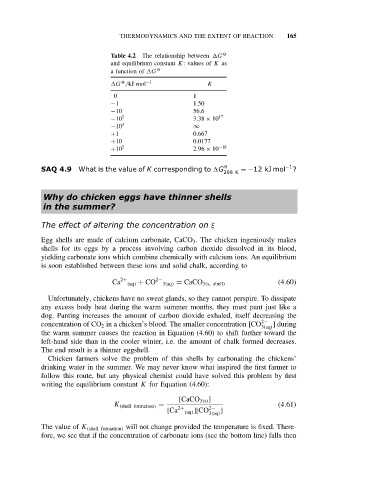
Table 4.2 The relationship between G O
and equilibrium constant K:valuesof K as
a function of G O
O
G /kJ mol −1 K
0 1
−1 1.50
−10 56.6
−10 2 3.38 × 10 17
−10 3 ∞
+1 0.667
+10 0.0177
+10 2 2.96 × 10 −18
SAQ 4.9 What is the value of K corresponding to G O =−12 kJ mol −1 ?
298 K
Why do chicken eggs have thinner shells
in the summer?
The effect of altering the concentration on ξ
Egg shells are made of calcium carbonate, CaCO 3 . The chicken ingeniously makes
shells for its eggs by a process involving carbon dioxide dissolved in its blood,
yielding carbonate ions which combine chemically with calcium ions. An equilibrium
is soon established between these ions and solid chalk, according to
Ca 2+ (aq) + CO 2− 3(aq) = CaCO 3(s, shell) (4.60)
Unfortunately, chickens have no sweat glands, so they cannot perspire. To dissipate
any excess body heat during the warm summer months, they must pant just like a
dog. Panting increases the amount of carbon dioxide exhaled, itself decreasing the
concentration of CO 2 in a chicken’s blood. The smaller concentration [CO 2− ] during
3(aq)
the warm summer causes the reaction in Equation (4.60) to shift further toward the
left-hand side than in the cooler winter, i.e. the amount of chalk formed decreases.
The end result is a thinner eggshell.
Chicken farmers solve the problem of thin shells by carbonating the chickens’
drinking water in the summer. We may never know what inspired the first farmer to
follow this route, but any physical chemist could have solved this problem by first
writing the equilibrium constant K for Equation (4.60):
[CaCO 3(s) ]
K (shell formation) = (4.61)
[Ca 2+ (aq) ][CO 2− ]
3(aq)
The value of K (shell formation) will not change provided the temperature is fixed. There-
fore, we see that if the concentration of carbonate ions (see the bottom line) falls then