Page 208 - Physical chemistry understanding our chemical world
P. 208
THE EFFECT OF TEMPERATURE ON THERMODYNAMIC VARIABLES 175
0.6
0.5
0.4
K
ln
0.3
0.2
0.1
0.0012 0.0013 0.0014 0.0015
1 ÷ (T / K)
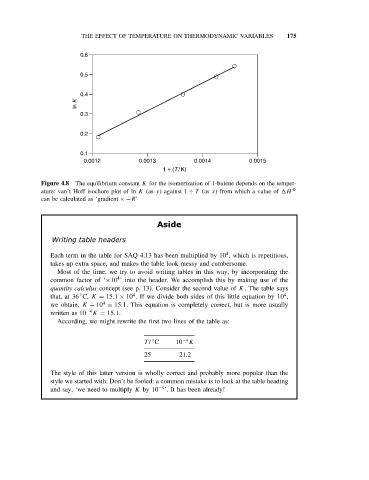
Figure 4.8 The equilibrium constant K for the isomerization of 1-butene depends on the temper-
ature: van’t Hoff isochore plot of ln K (as y)against 1 ÷ T (as x) from which a value of H O
can be calculated as ‘gradient ×−R’
Aside
Writing table headers
4
Each term in the table for SAQ 4.13 has been multiplied by 10 , which is repetitious,
takes up extra space, and makes the table look messy and cumbersome.
Most of the time, we try to avoid writing tables in this way, by incorporating the
4
common factor of ‘×10 ’ into the header. We accomplish this by making use of the
quantity calculus concept (see p. 13). Consider the second value of K. The table says
◦
4
4
that, at 36 C, K = 15.1 × 10 . If we divide both sides of this little equation by 10 ,
4
we obtain, K ÷ 10 = 15.1. This equation is completely correct, but is more usually
−4
written as 10 K = 15.1.
According, we might rewrite the first two lines of the table as:
−4
◦
T / C 10 K
25 21.2
The style of this latter version is wholly correct and probably more popular than the
style we started with. Don’t be fooled: a common mistake is to look at the table heading
−4
and say, ‘we need to multiply K by 10 ’. It has been already!