Page 42 - Physical chemistry understanding our chemical world
P. 42
THE PRACTICE OF THERMODYNAMIC MEASUREMENT 9
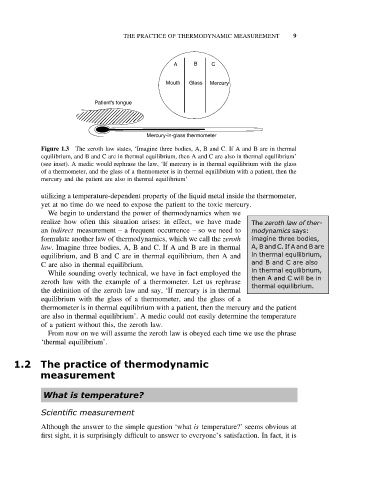
A B C
Mouth Glass Mercury
Patient's tongue
Mercury-in-glass thermometer
Figure 1.3 The zeroth law states, ‘Imagine three bodies, A, B and C. If A and B are in thermal
equilibrium, and B and C are in thermal equilibrium, then A and C are also in thermal equilibrium’
(see inset). A medic would rephrase the law, ‘If mercury is in thermal equilibrium with the glass
of a thermometer, and the glass of a thermometer is in thermal equilibrium with a patient, then the
mercury and the patient are also in thermal equilibrium’
utilizing a temperature-dependent property of the liquid metal inside the thermometer,
yet at no time do we need to expose the patient to the toxic mercury.
We begin to understand the power of thermodynamics when we
realize how often this situation arises: in effect, we have made The zeroth law of ther-
an indirect measurement – a frequent occurrence – so we need to modynamics says:
formulate another law of thermodynamics, which we call the zeroth imagine three bodies,
law. Imagine three bodies, A, B and C. If A and B are in thermal A, B and C. If A and B are
equilibrium, and B and C are in thermal equilibrium, then A and in thermal equilibrium,
C are also in thermal equilibrium. and B and C are also
in thermal equilibrium,
While sounding overly technical, we have in fact employed the
then A and C will be in
zeroth law with the example of a thermometer. Let us rephrase
thermal equilibrium.
the definition of the zeroth law and say, ‘If mercury is in thermal
equilibrium with the glass of a thermometer, and the glass of a
thermometer is in thermal equilibrium with a patient, then the mercury and the patient
are also in thermal equilibrium’. A medic could not easily determine the temperature
of a patient without this, the zeroth law.
From now on we will assume the zeroth law is obeyed each time we use the phrase
‘thermal equilibrium’.
1.2 The practice of thermodynamic
measurement
What is temperature?
Scientific measurement
Although the answer to the simple question ‘what is temperature?’ seems obvious at
first sight, it is surprisingly difficult to answer to everyone’s satisfaction. In fact, it is