Page 49 - Physical chemistry understanding our chemical world
P. 49
16 INTRODUCTION TO PHYSICAL CHEMISTRY
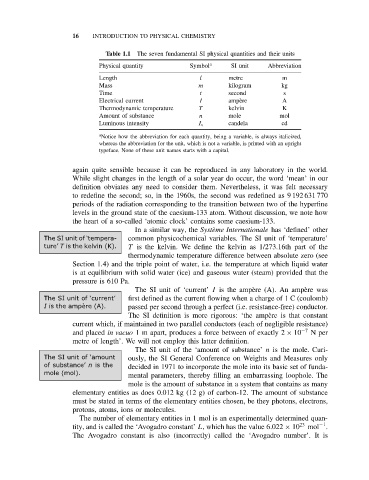
Table 1.1 The seven fundamental SI physical quantities and their units
Physical quantity Symbol a SI unit Abbreviation
Length l metre m
Mass m kilogram kg
Time t second s
Electrical current I amp` ere A
Thermodynamic temperature T kelvin K
Amount of substance n mole mol
Luminous intensity I v candela cd
a Notice how the abbreviation for each quantity, being a variable, is always italicized,
whereas the abbreviation for the unit, which is not a variable, is printed with an upright
typeface. None of these unit names starts with a capital.
again quite sensible because it can be reproduced in any laboratory in the world.
While slight changes in the length of a solar year do occur, the word ‘mean’ in our
definition obviates any need to consider them. Nevertheless, it was felt necessary
to redefine the second; so, in the 1960s, the second was redefined as 9 192 631 770
periods of the radiation corresponding to the transition between two of the hyperfine
levels in the ground state of the caesium-133 atom. Without discussion, we note how
the heart of a so-called ‘atomic clock’ contains some caesium-133.
In a similar way, the Syst`eme Internationale has ‘defined’ other
The SI unit of ‘tempera- common physicochemical variables. The SI unit of ‘temperature’
ture’ T is the kelvin (K). T is the kelvin. We define the kelvin as 1/273.16th part of the
thermodynamic temperature difference between absolute zero (see
Section 1.4) and the triple point of water, i.e. the temperature at which liquid water
is at equilibrium with solid water (ice) and gaseous water (steam) provided that the
pressure is 610 Pa.
The SI unit of ‘current’ I is the amp` ere (A). An amp` ere was
The SI unit of ‘current’ first defined as the current flowing when a charge of 1 C (coulomb)
I is the amp` ere (A). passed per second through a perfect (i.e. resistance-free) conductor.
The SI definition is more rigorous: ‘the amp` ere is that constant
current which, if maintained in two parallel conductors (each of negligible resistance)
and placed in vacuo 1 m apart, produces a force between of exactly 2 × 10 −7 N per
metre of length’. We will not employ this latter definition.
The SI unit of the ‘amount of substance’ n is the mole. Curi-
The SI unit of ‘amount ously, the SI General Conference on Weights and Measures only
of substance’ n is the decided in 1971 to incorporate the mole into its basic set of funda-
mole (mol). mental parameters, thereby filling an embarrassing loophole. The
mole is the amount of substance in a system that contains as many
elementary entities as does 0.012 kg (12 g) of carbon-12. The amount of substance
must be stated in terms of the elementary entities chosen, be they photons, electrons,
protons, atoms, ions or molecules.
The number of elementary entities in 1 mol is an experimentally determined quan-
−1
tity, and is called the ‘Avogadro constant’ L, which has the value 6.022 × 10 23 mol .
The Avogadro constant is also (incorrectly) called the ‘Avogadro number’. It is