Page 51 - Physical chemistry understanding our chemical world
P. 51
18 INTRODUCTION TO PHYSICAL CHEMISTRY
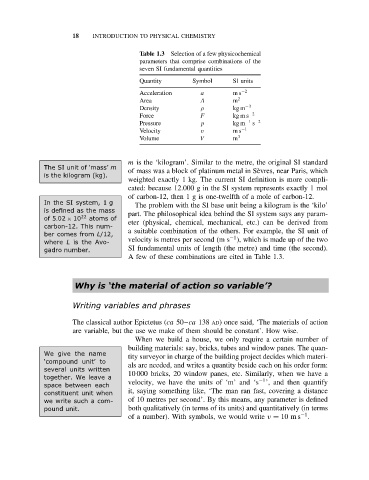
Table 1.3 Selection of a few physicochemical
parameters that comprise combinations of the
seven SI fundamental quantities
Quantity Symbol SI units
Acceleration a ms −2
Area A m 2
Density ρ kg m −3
Force F kg m s −2
s
Pressure p kg m −1 −2
Velocity v ms −1
Volume V m 3
m is the ‘kilogram’. Similar to the metre, the original SI standard
The SI unit of ‘mass’ m
is the kilogram (kg). of mass was a block of platinum metal in S` evres, near Paris, which
weighted exactly 1 kg. The current SI definition is more compli-
cated: because 12.000 g in the SI system represents exactly 1 mol
of carbon-12, then 1 g is one-twelfth of a mole of carbon-12.
In the SI system, 1 g The problem with the SI base unit being a kilogram is the ‘kilo’
is defined as the mass part. The philosophical idea behind the SI system says any param-
of 5.02 × 10 22 atoms of eter (physical, chemical, mechanical, etc.) can be derived from
carbon-12. This num-
a suitable combination of the others. For example, the SI unit of
ber comes from L/12, −1
where L is the Avo- velocity is metres per second (m s ), which is made up of the two
gadro number. SI fundamental units of length (the metre) and time (the second).
A few of these combinations are cited in Table 1.3.
Why is ‘the material of action so variable’?
Writing variables and phrases
The classical author Epictetus (ca 50–ca 138 AD) once said, ‘The materials of action
are variable, but the use we make of them should be constant’. How wise.
When we build a house, we only require a certain number of
building materials: say, bricks, tubes and window panes. The quan-
We give the name tity surveyor in charge of the building project decides which materi-
‘compound unit’ to als are needed, and writes a quantity beside each on his order form:
several units written
together. We leave a 10 000 bricks, 20 window panes, etc. Similarly, when we have a
−1
space between each velocity, we have the units of ‘m’ and ‘s ’, and then quantify
constituent unit when it, saying something like, ‘The man ran fast, covering a distance
we write such a com- of 10 metres per second’. By this means, any parameter is defined
pound unit. both qualitatively (in terms of its units) and quantitatively (in terms
−1
of a number). With symbols, we would write v = 10 m s .