Page 17 - Physical chemistry eng
P. 17
xvi PREFACE
• Integrate computational chemistry into the standard curriculum. The teaching of
quantum mechanics has not taken advantage of the widespread availability of Ab Initio
Software. Many chapters include computational problems for which detailed instruc-
®
tions for the student are available in the Study Area in MasteringChemistry . It is our
experience that students welcome this material, (see L. Johnson and T. Engel, Journal of
Chemical Education 2011, 88 [569-573]) which transforms the teaching of chemical
bonding and molecular structure from being qualitative to quantitative. For example, an
electrostatic potential map of acetonitrile built in Spartan Student is shown here.
• Key equations. Physical chemistry is a chemistry subdiscipline that is mathemat-
ics intensive in nature. Key equations that summarize fundamental relationships
between variables are colored in red for emphasis.
• Green boxes. Fundamental principles such as the laws of thermodynamics and
the quantum mechanical postulates are displayed in green boxes.
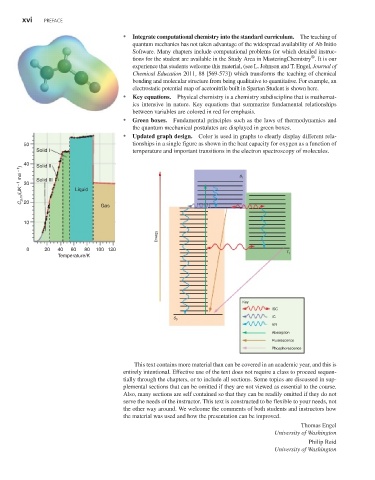
• Updated graph design. Color is used in graphs to clearly display different rela-
50 tionships in a single figure as shown in the heat capacity for oxygen as a function of
Solid I temperature and important transitions in the electron spectroscopy of molecules.
40 Solid II
mol 1 ) 30 Solid III S 1
C p,m /(JK 1 Liquid
20
Gas
10
Energy
0 20 40 60 80 100 120
T 1
Temperature/K
Key
ISC
IC
S 0
VR
Absorption
Fluorescence
Phosphorescence
This text contains more material than can be covered in an academic year, and this is
entirely intentional. Effective use of the text does not require a class to proceed sequen-
tially through the chapters, or to include all sections. Some topics are discussed in sup-
plemental sections that can be omitted if they are not viewed as essential to the course.
Also, many sections are self contained so that they can be readily omitted if they do not
serve the needs of the instructor. This text is constructed to be flexible to your needs, not
the other way around. We welcome the comments of both students and instructors how
the material was used and how the presentation can be improved.
Thomas Engel
University of Washington
Philip Reid
University of Washington