Page 14 - Physical chemistry eng
P. 14
Preface
The third edition of this book builds on user and reviewer comments on the previous
editions. Our goal remains to provide students with an accessible overview of the
whole field of physical chemistry while focusing on basic principles that unite
the subdisciplines of the field. We continue to present new research developments in
the field to emphasize the vibrancy of physical chemistry. Many chapters have been
extensively revised as described below. We include additional end-of-chapter concept
problems and most of the numerical problems have been revised. The target audience
remains undergraduate students majoring in chemistry, biochemistry, and chemical
engineering, as well as many students majoring in the atmospheric sciences and the
biological sciences. The following objectives, illustrated with brief examples, outline
our approach to teaching physical chemistry.
• Focus on teaching core concepts. The central principles of physical chemistry
are explored by focusing on core ideas, and then extending these ideas to a variety
of problems. The goal is to build a solid foundation of student understanding rather
than cover a wide variety of topics in modest detail.
• Illustrate the relevance of physical chemistry to the world around us. Many
students struggle to connect physical chemistry concepts to the world around them.
To address this issue, example problems and specific topics are tied together to help
the student develop this connection. Fuel cells, refrigerators, heat pumps, and real
engines are discussed in connection with the second law of thermodynamics. The
particle in the box model is used to explain why metals conduct electricity and why
valence electrons rather than core electrons are important in chemical bond forma-
tion. Examples are used to show the applications of chemical spectroscopies. Every
attempt is made to connect fundamental ideas to applications that are familiar to the
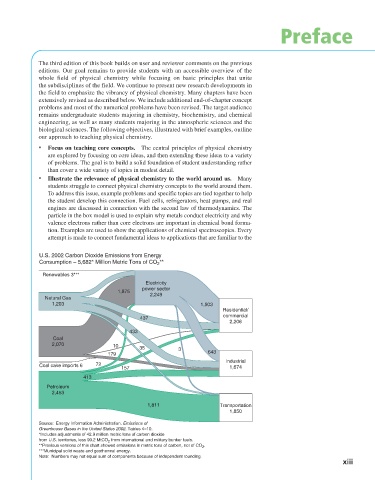
U.S. 2002 Carbon Dioxide Emissions from Energy
**
Consumption – 5,682* Million Metric Tons of CO 2
Renewables 3***
Electricity
power sector
1,875
2,249
Natural Gas
1,203 1,503
Residential/
commercial
437
2,206
433
Coal
2,070 10
35 3
179 643
Industrial
Coal cake imports 6 72
157 1,674
413
Petroleum
2,453
1,811 Transportation
1,850
Source: Energy Information Administration. Emissions of
Greenhouse Gases in the United States 2002. Tables 4–10.
*Includes adjustments of 42.9 million metric tons of carbon dioxide
from U.S. territories, less 90.2 MtCO from international and military bunker fuels.
2
**Previous versions of this chart showed emissions in metric tons of carbon, not of CO .
2
***Municipal solid waste and geothermal energy.
Note: Numbers may not equal sum of components because of independent rounding.
xiii