Page 55 - Physical chemistry eng
P. 55
32 CHAPTER 2 Heat, Work, Internal Energy, Enthalpy, and the First Law of Thermodynamics
P The magnitudes of the work in the forward and reverse processes are equal. The
25 i
total work done in this cyclical process is given by
V 2 V 1
w = w + w =-nRTln - nRTln
20 expansion compression V 1 V 2
V 2 V 2
=-nRTln + nRT ln = 0 (2.22)
15 V 1 V 1
P/bar Therefore, the work done in a reversible isothermal cycle is zero. Because
10 ¢U = q + w is a state function, q =-w = 0 for this reversible isothermal process.
Looking at the heights of the weights in the surroundings at the end of the process, we
find that they are the same as at the beginning of the process. To compare the work for
5 P f
reversible and irreversible processes, the state variables need to be given specific values
as is done in Example Problem 2.4.
0 5 10 15 20 25
V/L EXAMPLE PROBLEM 2.4
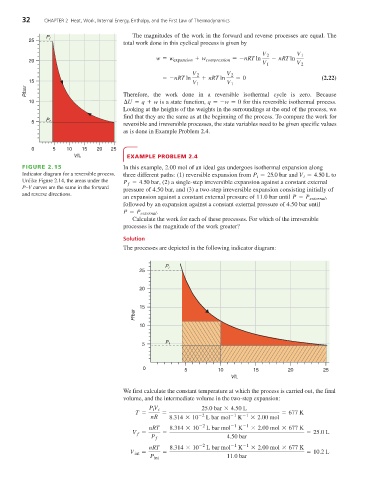
FIGURE 2.15 In this example, 2.00 mol of an ideal gas undergoes isothermal expansion along
Indicator diagram for a reversible process. three different paths: (1) reversible expansion from P = 25.0 bar and V = 4.50 L to
i
i
Unlike Figure 2.14, the areas under the P = 4.50 bar , (2) a single-step irreversible expansion against a constant external
f
P–V curves are the same in the forward
pressure of 4.50 bar, and (3) a two-step irreversible expansion consisting initially of
and reverse directions.
an expansion against a constant external pressure of 11.0 bar until P = P external ,
followed by an expansion against a constant external pressure of 4.50 bar until
P = P external .
Calculate the work for each of these processes. For which of the irreversible
processes is the magnitude of the work greater?
Solution
The processes are depicted in the following indicator diagram:
P i
25
20
15
P/bar
10
P
5 f
0 5 10 15 20 25
V/L
We first calculate the constant temperature at which the process is carried out, the final
volume, and the intermediate volume in the two-step expansion:
P V 25.0 bar * 4.50 L
i i
T = = = 677 K
-1
-2
nR 8.314 * 10 L bar mol K -1 * 2.00 mol
-2
-1
nRT 8.314 * 10 L bar mol K -1 * 2.00 mol * 677 K
V = = = 25.0 L
f
P f 4.50 bar
-2
-1
nRT 8.314 * 10 L bar mol K -1 * 2.00 mol * 677 K
= =
V int = 10.2 L
P int 11.0 bar