Page 56 - Physical chemistry eng
P. 56
2.8 COMPARING WORK FOR REVERSIBLE AND IRREVERSIBLE PROCESSES 33
The work of the reversible process is given by
V f
w =-nRT ln
1
V i
25.0 L 3
-1
-1
=-2.00 mol * 8.314 J mol K * 677 K * ln =-19.3 * 10 J
4.50 L
We next calculate the work of the single-step and two-step irreversible processes:
5
-3
10 Pa 10 m 3
w single =-P external ¢V =-4.50 bar * * (25.0 L - 4.50 L) *
bar L
3
=-9.23 * 10 J
5
-3
10 Pa 10 m 3
w two-step =-P external ¢V =-11.0 bar * * (10.2 L - 4.50 L) *
bar L
5
-3
10 Pa 10 m 3
-4.50 bar * * (25.0 L - 10.2 L) *
bar L
3
=-12.9 * 10 J
The magnitude of the work is greater for the two-step process than for the single-step
process, but less than that for the reversible process.
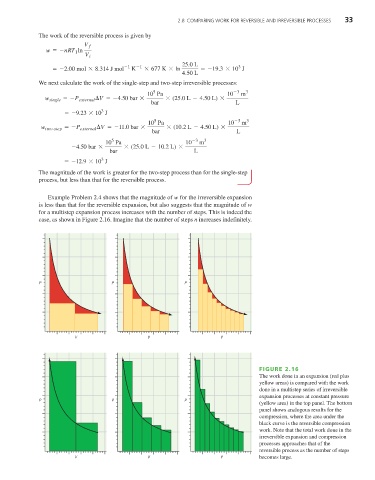
Example Problem 2.4 shows that the magnitude of w for the irreversible expansion
is less than that for the reversible expansion, but also suggests that the magnitude of w
for a multistep expansion process increases with the number of steps. This is indeed the
case, as shown in Figure 2.16. Imagine that the number of steps n increases indefinitely.
P P P
V V V
FIGURE 2.16
The work done in an expansion (red plus
yellow areas) is compared with the work
done in a multistep series of irreversible
expansion processes at constant pressure
P P P
(yellow area) in the top panel. The bottom
panel shows analogous results for the
compression, where the area under the
black curve is the reversible compression
work. Note that the total work done in the
irreversible expansion and compression
processes approaches that of the
reversible process as the number of steps
V V V becomes large.