Page 233 - Physical Chemistry
P. 233
lev38627_ch07.qxd 3/14/08 12:51 PM Page 214
214
Chapter 7 TABLE 7.1
One-Component Phase Equilibrium
and Surfaces
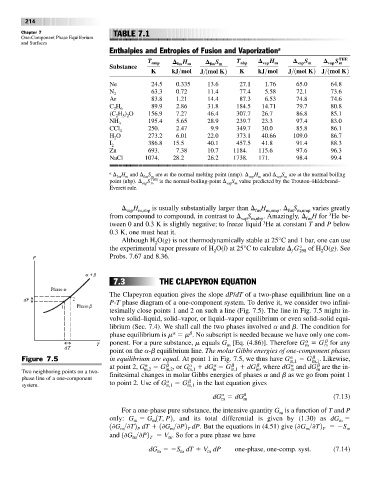
Enthalpies and Entropies of Fusion and Vaporization a
THE
T nmp fus H m fus S m T nbp vap H m vap S m vap S m
Substance
K kJ>mol J>1mol K2 K kJ>mol J>1mol K2 J>1mol K2
Ne 24.5 0.335 13.6 27.1 1.76 65.0 64.8
N 2 63.3 0.72 11.4 77.4 5.58 72.1 73.6
Ar 83.8 1.21 14.4 87.3 6.53 74.8 74.6
C H 6 89.9 2.86 31.8 184.5 14.71 79.7 80.8
2
(C H ) O 156.9 7.27 46.4 307.7 26.7 86.8 85.1
5 2
2
NH 3 195.4 5.65 28.9 239.7 23.3 97.4 83.0
CCl 4 250. 2.47 9.9 349.7 30.0 85.8 86.1
H O 273.2 6.01 22.0 373.1 40.66 109.0 86.7
2
I 2 386.8 15.5 40.1 457.5 41.8 91.4 88.3
Zn 693. 7.38 10.7 1184. 115.6 97.6 96.3
NaCl 1074. 28.2 26.2 1738. 171. 98.4 99.4
a H and S are at the normal melting point (nmp). H and S are at the normal boiling
vap m
fus
m
vap
m
fus m
point (nbp). S THE is the normal-boiling-point S value predicted by the Trouton–Hildebrand–
vap m
vap m
Everett rule.
H is usually substantially larger than H . S varies greatly
vap m,nbp fus m,nmp fus m,nmp
3
from compound to compound, in contrast to S . Amazingly, H for He be-
vap m,nbp fus
3
tween 0 and 0.3 K is slightly negative; to freeze liquid He at constant T and P below
0.3 K, one must heat it.
Although H O(g) is not thermodynamically stable at 25°C and 1 bar, one can use
2
the experimental vapor pressure of H O(l) at 25°C to calculate G° of H O(g). See
2 f 298 2
Probs. 7.67 and 8.36.
7.3 THE CLAPEYRON EQUATION
The Clapeyron equation gives the slope dP/dT of a two-phase equilibrium line on a
P-T phase diagram of a one-component system. To derive it, we consider two infini-
tesimally close points 1 and 2 on such a line (Fig. 7.5). The line in Fig. 7.5 might in-
volve solid–liquid, solid–vapor, or liquid–vapor equilibrium or even solid–solid equi-
librium (Sec. 7.4). We shall call the two phases involved a and b. The condition for
b
a
phase equilibrium is m m . No subscript is needed because we have only one com-
b
a
ponent. For a pure substance, m equals G [Eq. (4.86)]. Therefore G G for any
m m m
point on the a-b equilibrium line. The molar Gibbs energies of one-component phases
a
Figure 7.5 in equilibrium are equal. At point 1 in Fig. 7.5, we thus have G m,1 G b . Likewise,
m,1
b
b
a
b
a
b
a
a
at point 2, G m,2 G m,2 , or G m,1 dG G m,1 dG , where dG and dG are the in-
m
m
m
m
Two neighboring points on a two- finitesimal changes in molar Gibbs energies of phases a and b as we go from point 1
phase line of a one-component a b
system. to point 2. Use of G m,1 G m,1 in the last equation gives
a
dG dG b m (7.13)
m
For a one-phase pure substance, the intensive quantity G is a function of T and P
m
only: G G 1T, P2, and its total differential is given by (1.30) as dG
m
m
m
10G >0T2 dT 10G >0P2 dP. But the equations in (4.51) give 10G >0T2 S m
m
P
m
P
T
m
and 10G >0P2 V . So for a pure phase we have
m
T
m
dG S dT V dP one-phase, one-comp. syst. (7.14)
m
m
m