Page 247 - Physical Chemistry
P. 247
lev38627_ch07.qxd 3/14/08 12:51 PM Page 228
228
Chapter 7 V W
One-Component Phase Equilibrium
and Surfaces
A B
C D
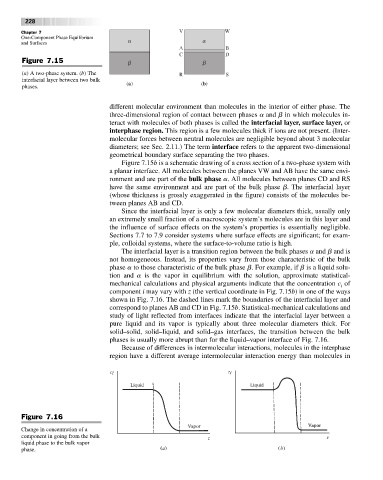
Figure 7.15
(a) A two-phase system. (b) The R S
interfacial layer between two bulk
phases. (a) (b)
different molecular environment than molecules in the interior of either phase. The
three-dimensional region of contact between phases a and b in which molecules in-
teract with molecules of both phases is called the interfacial layer, surface layer, or
interphase region. This region is a few molecules thick if ions are not present. (Inter-
molecular forces between neutral molecules are negligible beyond about 3 molecular
diameters; see Sec. 2.11.) The term interface refers to the apparent two-dimensional
geometrical boundary surface separating the two phases.
Figure 7.15b is a schematic drawing of a cross section of a two-phase system with
a planar interface. All molecules between the planes VW and AB have the same envi-
ronment and are part of the bulk phase a. All molecules between planes CD and RS
have the same environment and are part of the bulk phase b. The interfacial layer
(whose thickness is grossly exaggerated in the figure) consists of the molecules be-
tween planes AB and CD.
Since the interfacial layer is only a few molecular diameters thick, usually only
an extremely small fraction of a macroscopic system’s molecules are in this layer and
the influence of surface effects on the system’s properties is essentially negligible.
Sections 7.7 to 7.9 consider systems where surface effects are significant; for exam-
ple, colloidal systems, where the surface-to-volume ratio is high.
The interfacial layer is a transition region between the bulk phases a and b and is
not homogeneous. Instead, its properties vary from those characteristic of the bulk
phase a to those characteristic of the bulk phase b. For example, if b is a liquid solu-
tion and a is the vapor in equilibrium with the solution, approximate statistical-
mechanical calculations and physical arguments indicate that the concentration c of
i
component i may vary with z (the vertical coordinate in Fig. 7.15b) in one of the ways
shown in Fig. 7.16. The dashed lines mark the boundaries of the interfacial layer and
correspond to planes AB and CD in Fig. 7.15b. Statistical-mechanical calculations and
study of light reflected from interfaces indicate that the interfacial layer between a
pure liquid and its vapor is typically about three molecular diameters thick. For
solid–solid, solid–liquid, and solid–gas interfaces, the transition between the bulk
phases is usually more abrupt than for the liquid–vapor interface of Fig. 7.16.
Because of differences in intermolecular interactions, molecules in the interphase
region have a different average intermolecular interaction energy than molecules in
Figure 7.16
Change in concentration of a
component in going from the bulk
liquid phase to the bulk vapor
phase.