Page 394 - Physical Chemistry
P. 394
lev38627_ch12.qxd 3/18/08 2:41 PM Page 375
375
mixture that has the eutectic composition will melt entirely at one temperature (T ). Section 12.8
3
Two-Component
A solution of B and C that has the eutectic composition will freeze entirely at tem- Solid–Liquid Equilibrium
perature T to produce a eutectic mixture of solids B and C. However, a eutectic mix-
3
ture is not a compound. Microscopic examination will show the eutectic solid to be an
intimate mixture of crystals of B and crystals of C.
Systems with the solid–liquid phase diagram of Fig. 12.19 are called simple
eutectic systems. Examples include Pb–Sb, benzene–naphthalene, Si–Al, KCl–AgCl,
Bi–Cd, C H –CH Cl, and chloroform–aniline.
6
6
3
Solid Solutions
Certain pairs of substances form solid solutions. In a solid solution of B and C, there
are no individual crystals of B or C. Instead, the molecules or atoms or ions are mixed
together at the molecular level, and the composition of the solution can be varied con-
tinuously over a certain range. Solid solutions can be prepared by condensing a vapor
of B plus C or by cooling a liquid solution of B and C. Two solids might be completely
miscible, partly miscible, or completely immiscible.
In an interstitial solid solution, the B molecules or atoms (which must be small)
occupy interstices (holes) in the crystal structure of substance C. For example, steel is
a solution in which carbon atoms occupy interstices in the Fe crystal structure. In a
substitutional solid solution, molecules or atoms or ions of B substitute for those of C
at random locations in the crystal structure. Examples include Cu–Ni, Na CO –
2
3
K CO , and p-dichlorobenzene–p-dibromobenzene. Substitutional solids are formed
2
3
by substances with atoms, molecules, or ions of similar size and structure.
Analysis of a transition-metal oxide or sulfide frequently shows an apparent vio-
lation of the law of definite proportions. For example, ZnO usually has a Zn/O mole
ratio slightly greater than 1. The explanation is that the “zinc oxide” is actually an
interstitial solid solution of Zn in ZnO.
Liquid-Phase Miscibility and Solid-Phase Miscibility
Some pairs of substances are completely miscible in the solid state. Examples include
Cu–Ni, Sb–Bi, Pd–Ni, KNO –NaNO , and d-carvoxime–l-carvoxime. With complete
3
3
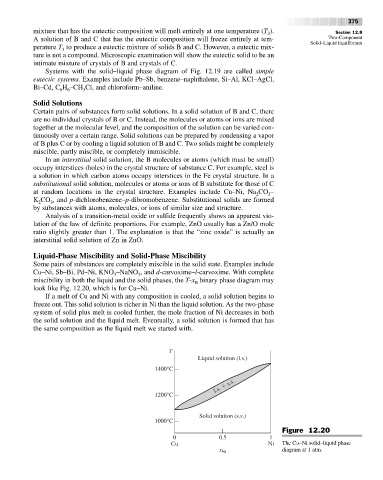
miscibility in both the liquid and the solid phases, the T-x binary phase diagram may
B
look like Fig. 12.20, which is for Cu–Ni.
If a melt of Cu and Ni with any composition is cooled, a solid solution begins to
freeze out. This solid solution is richer in Ni than the liquid solution. As the two-phase
system of solid plus melt is cooled further, the mole fraction of Ni decreases in both
the solid solution and the liquid melt. Eventually, a solid solution is formed that has
the same composition as the liquid melt we started with.
T
Liquid solution (l.s.)
1400°C
l.s. s.s.
1200°C
Solid solution (s.s.)
1000°C
Figure 12.20
0 0.5 1
Cu Ni The Cu–Ni solid–liquid phase
diagram at 1 atm.
x Ni