Page 63 - Physical Chemistry
P. 63
lev38627_ch02.qxd 2/29/08 3:11 PM Page 44
44
Chapter 2 Line Integrals
The First Law of Thermodynamics The integral PdV in (2.27) is not an ordinary integral. For a closed system of
2
1
fixed composition, the system’s pressure P is a function of its temperature and volume:
P P(T, V). To calculate w , we must evaluate the negative of
rev
2
P1T, V2 dV (2.28)
1
The integrand P(T, V) is a function of two independent variables T and V. In an ordinary
definite integral, the integrand is a function of one variable, and the value of the ordinary
b
definite integral f(x) dx is determined once the function f and the limits a and b are
a
2
3
3
3
2
specified. For example, x dx 3 /3 1 /3 26/3. In contrast, in P(T, V) dV,
1 1
both of the independent variables T and V may change during the volume-change
process, and the value of the integral depends on how T and V vary. For example, if the
2
2
system is an ideal gas, then P nRT/V and P(T, V) dV nR (T/V) dV. Before we
1 1
2
can evaluate (T/V) dV, we must know how both T and V change during the process.
1
P The integral (2.28) is called a line integral. Sometimes the letter L is put under
the integral sign of a line integral. The value of the line integral (2.28) is defined as
1
the sum of the infinitesimal quantities P(T, V) dV for the particular process used to go
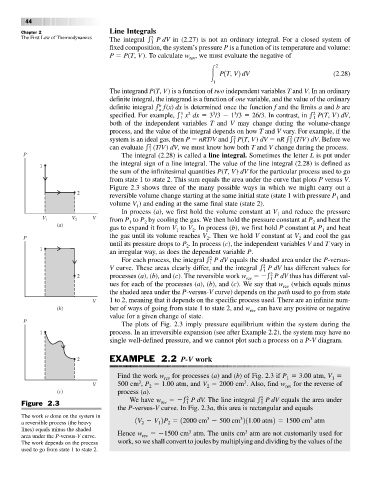
from state 1 to state 2. This sum equals the area under the curve that plots P versus V.
Figure 2.3 shows three of the many possible ways in which we might carry out a
2
reversible volume change starting at the same initial state (state 1 with pressure P and
1
volume V ) and ending at the same final state (state 2).
1
In process (a), we first hold the volume constant at V and reduce the pressure
1
V 1 V 2 V from P to P by cooling the gas. We then hold the pressure constant at P and heat the
(a) 1 2 2
gas to expand it from V to V . In process (b), we first hold P constant at P and heat
1 2 1
the gas until its volume reaches V . Then we hold V constant at V and cool the gas
P 2 2
until its pressure drops to P . In process (c), the independent variables V and T vary in
2
1
an irregular way, as does the dependent variable P.
2
For each process, the integral P dV equals the shaded area under the P-versus-
1
2
V curve. These areas clearly differ, and the integral PdV has different values for
1
2
2 processes (a), (b), and (c). The reversible work w PdV thus has different val-
rev 1
ues for each of the processes (a), (b), and (c). We say that w (which equals minus
rev
the shaded area under the P-versus-V curve) depends on the path used to go from state
V 1 to 2, meaning that it depends on the specific process used. There are an infinite num-
(b) ber of ways of going from state 1 to state 2, and w can have any positive or negative
rev
value for a given change of state.
P
The plots of Fig. 2.3 imply pressure equilibrium within the system during the
1 process. In an irreversible expansion (see after Example 2.2), the system may have no
single well-defined pressure, and we cannot plot such a process on a P-V diagram.
2 EXAMPLE 2.2 P-V work
Find the work w for processes (a) and (b) of Fig. 2.3 if P 3.00 atm, V
rev 1 1
3
3
V 500 cm , P 1.00 atm, and V 2000 cm . Also, find w rev for the reverse of
2
2
(c) process (a).
2
2
We have w PdV. The line integral PdV equals the area under
Figure 2.3 rev 1 1
the P-versus-V curve. In Fig. 2.3a, this area is rectangular and equals
The work w done on the system in 3 3 3
a reversible process (the heavy 1V V 2P 12000 cm 500 cm 211.00 atm2 1500 cm atm
2
1
2
lines) equals minus the shaded 3 3
area under the P-versus-V curve. Hence w rev 1500 cm atm. The units cm atm are not customarily used for
The work depends on the process work, so we shall convert to joules by multiplying and dividing by the values of the
used to go from state 1 to state 2.