Page 248 - Pipeline Pigging Technology
P. 248
Pigging and chemical treatment
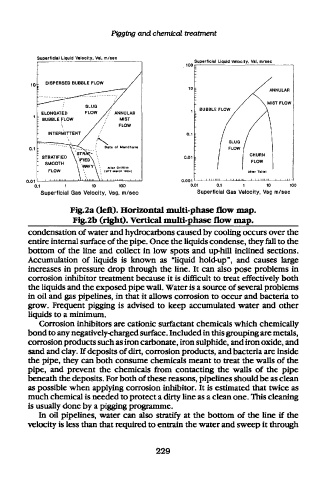
Fig.2a (left). Horizontal multi-phase flow map.
Fig.2b (right). Vertical multi-phase flow map.
condensation of water and hydrocarbons caused by cooling occurs over the
entire internal surface of the pipe. Once the liquids condense, they fall to the
bottom of the line and collect in low spots and up-hill inclined sections.
Accumulation of liquids is known as "liquid hold-up", and causes large
increases in pressure drop through the line. It can also pose problems in
corrosion inhibitor treatment because it is difficult to treat effectively both
the liquids and the exposed pipe wall. Water is a source of several problems
in oil and gas pipelines, in that it allows corrosion to occur and bacteria to
grow. Frequent pigging is advised to keep accumulated water and other
liquids to a minimum.
Corrosion inhibitors are cationic surfactant chemicals which chemically
bond to any negatively-charged surface. Included in this grouping are metals,
corrosion products such as iron carbonate, iron sulphide, and iron oxide, and
sand and clay. If deposits of dirt, corrosion products, and bacteria are inside
the pipe, they can both consume chemicals meant to treat the walls of the
pipe, and prevent the chemicals from contacting the walls of the pipe
beneath the deposits. For both of these reasons, pipelines should be as clean
as possible when applying corrosion inhibitor. It is estimated that twice as
much chemical is needed to protect a dirty line as a clean one. This cleaning
is usually done by a pigging programme.
In oil pipelines, water can also stratify at the bottom of the line if the
velocity is less than that required to entrain the water and sweep it through
229