Page 216 - Pipeline Rules of Thumb Handbook
P. 216
Corrosion/Coatings 203
sulfate cell is -1.1 volts; standard magnesium has a solution Magnesium anodes may be consumed by self corrosion if
potential of -1.55 volts; and high purity magnesium has a operated at very low current densities. Refer to Figures
solution potential of -1.8 volts. 7a, 7b, 8a, and 8b for zinc anode performance data. The
If, for example, a pipeline is protected with zinc anodes at data in Figures 7a and 7b are based on the anodes being
a polarization potential of -0.9 volts, the driving potential will installed in a gypsum-clay backfill and having a driving poten-
be -1.1 - (-0.9) or -0.2 volts. If standard magnesium is used, tial of -0.2 volts. Figures 8a and 8b are based on the anodes
the driving potential will be -1.55 - (-0.9) or -0.65 volts. The being installed in water and having a driving potential of
circuit resistance for magnesium will be approximately three -0.2 volts. 1
times as great as for zinc. This would be handled by using
fewer magnesium anodes, smaller anodes, or using series
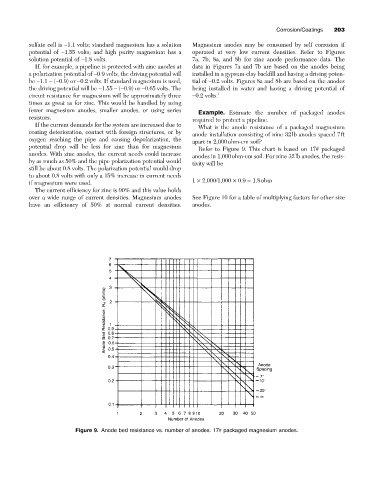
Example. Estimate the number of packaged anodes
resistors.
required to protect a pipeline.
If the current demands for the system are increased due to
What is the anode resistance of a packaged magnesium
coating deterioration, contact with foreign structures, or by
anode installation consisting of nine 32lb anodes spaced 7ft
oxygen reaching the pipe and causing depolarization, the
apart in 2,000ohm-cm soil?
potential drop will be less for zinc than for magnesium
Refer to Figure 9. This chart is based on 17# packaged
anodes. With zinc anodes, the current needs could increase
anodes in 1,000ohm-cm soil. For nine 32lb anodes, the resis-
by as much as 50% and the pipe polarization potential would
tivity will be
still be about 0.8 volts. The polarization potential would drop
to about 0.8 volts with only a 15% increase in current needs
if magnesium were used. 1 ¥ 2,000/1,000 ¥ 0.9 = 1.8ohm
The current efficiency for zinc is 90% and this value holds
over a wide range of current densities. Magnesium anodes See Figure 10 for a table of multiplying factors for other size
have an efficiency of 50% at normal current densities. anodes.
Figure 9. Anode bed resistance vs. number of anodes. 17# packaged magnesium anodes.