Page 131 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 131
Energy and environmental applications of graphene and its derivatives 107
and exciting applications, following behind CNTs and fullerene with some
advantageous features involving unique electronic quality, high crystal, and some
advantageous features [2,3]. Graphite is proposed to be derived from graphene sheets
stacked on top of each other (interlayer distance of 3.37°A); “fullerenes” and “CNTs”
are predicted to be made by wrapping and rolling a section of a 2-D graphene sheet [2].
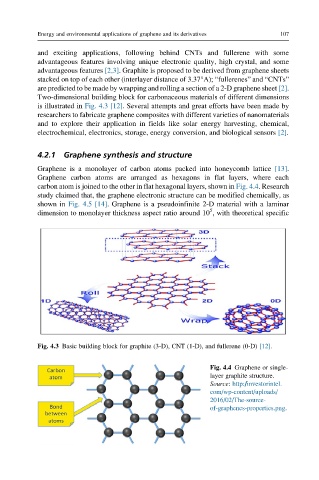
Two-dimensional building block for carbonaceous materials of different dimensions
is illustrated in Fig. 4.3 [12]. Several attempts and great efforts have been made by
researchers to fabricate graphene composites with different varieties of nanomaterials
and to explore their application in fields like solar energy harvesting, chemical,
electrochemical, electronics, storage, energy conversion, and biological sensors [2].
4.2.1 Graphene synthesis and structure
Graphene is a monolayer of carbon atoms packed into honeycomb lattice [13].
Graphene carbon atoms are arranged as hexagons in flat layers, where each
carbon atom is joined to the other in flat hexagonal layers, shown in Fig. 4.4. Research
study claimed that, the graphene electronic structure can be modified chemically, as
shown in Fig. 4.5 [14]. Graphene is a pseudoinfinite 2-D material with a laminar
5
dimension to monolayer thickness aspect ratio around 10 , with theoretical specific
Fig. 4.3 Basic building block for graphite (3-D), CNT (1-D), and fullerene (0-D) [12].
Fig. 4.4 Graphene or single-
layer graphite structure.
Source: http://investorintel.
com/wp-content/uploads/
2016/02/The-source-
of-graphenes-properties.png.