Page 136 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 136
Energy and environmental applications of graphene and its derivatives 111
4.2.2 Graphene properties
Graphene is the crystalline, strongest and stiffest nanomaterial ever identified [22],
with its freestanding monolayer exhibiting salient nonlinear elastic behavior [14].
It is a 2-D allotrope of carbon characterized with specific combination of properties
2 1
like extremely high specific surface area of 2630 m g even larger than other
carbon-based materials, due to high aspect ratio, remarkable electron mobility
2
s ), resistivity of 10
at room temperature (15,000 cm V 1 1 6 Ω cm, and gapless
semiconductor [21]. The perfect 2-D crystal quality also directs to superior thermal
conductivity of approximately 5300 W m 1 K 1 at room temperature, relatively
50% higher than CNTs, and 10 times higher aluminum and copper metals [27]. Incred-
ibly higher conductivity was explained on account of the presence of electron
cloud above and below each carbon ring, which overlaps to create a continuous pi
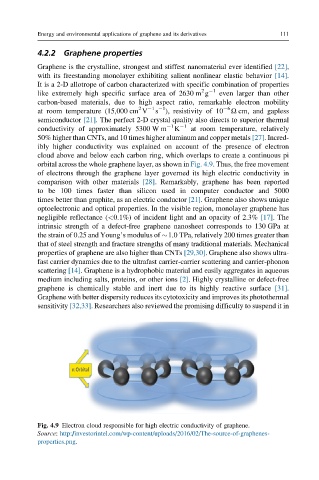
orbital across the whole graphene layer, as shown in Fig. 4.9. Thus, the free movement
of electrons through the graphene layer governed its high electric conductivity in
comparison with other materials [28]. Remarkably, graphene has been reported
to be 100 times faster than silicon used in computer conductor and 5000
times better than graphite, as an electric conductor [21]. Graphene also shows unique
optoelectronic and optical properties. In the visible region, monolayer graphene has
negligible reflectance (<0.1%) of incident light and an opacity of 2.3% [17]. The
intrinsic strength of a defect-free graphene nanosheet corresponds to 130 GPa at
the strain of 0.25 and Young’s modulus of 1.0 TPa, relatively 200 times greater than
that of steel strength and fracture strengths of many traditional materials. Mechanical
properties of graphene are also higher than CNTs [29,30]. Graphene also shows ultra-
fast carrier dynamics due to the ultrafast carrier-carrier scattering and carrier-phonon
scattering [14]. Graphene is a hydrophobic material and easily aggregates in aqueous
medium including salts, proteins, or other ions [2]. Highly crystalline or defect-free
graphene is chemically stable and inert due to its highly reactive surface [31].
Graphene with better dispersity reduces its cytotoxicity and improves its photothermal
sensitivity [32,33]. Researchers also reviewed the promising difficulty to suspend it in
Fig. 4.9 Electron cloud responsible for high electric conductivity of graphene.
Source: http://investorintel.com/wp-content/uploads/2016/02/The-source-of-graphenes-
properties.png.