Page 139 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 139
114 Polymer-based Nanocomposites for Energy and Environmental Applications
4.4.1 Lithium ion batteries (LIBs)
Graphene in the form of 3-D graphite electrodes has currently been used in LIB
technology as a key source of power for portable electronic appliances, especially
wireless telephones, computers, laptop, and digital cameras from past 20 years,
along with LiCoO 2 electrodes [10]. Three-dimensional graphene also represents the
dominant commercial anode material, however, not suitable as a practical anode
1
for hybrid EVs and HEVs, owing to its low theoretical capacity (372 mAh g ). Dop-
ing of S, B, or N atoms into graphene planes perfectly improves its electron transport
ability along with electrochemical performance [49,50]. Recently, fabrication of
anode materials composed of GBM composites of electrochemically active materials,
doped graphene, and graphene shows immense applications in supercapacitors
and fuel cells [10]. Researchers also claimed that 3-D graphene with hole and pores
possesses high specific surface area, and they remarkably offer abundant accessible
channels for lithium-ion transport [51,52]. Three-dimensional graphene-based
composites were fabricated to expand the electrochemical properties and conductivity
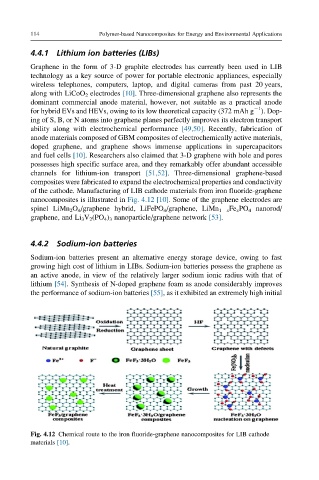
of the cathode. Manufacturing of LIB cathode materials from iron fluoride-graphene
nanocomposites is illustrated in Fig. 4.12 [10]. Some of the graphene electrodes are
spinel LiMn 2 O 4 /graphene hybrid, LiFePO 4 /graphene, LiMn 1 x Fe x PO 4 nanorod/
graphene, and Li 3 V 2 (PO 4 ) 3 nanoparticle/graphene network [53].
4.4.2 Sodium-ion batteries
Sodium-ion batteries present an alternative energy storage device, owing to fast
growing high cost of lithium in LIBs. Sodium-ion batteries possess the graphene as
an active anode, in view of the relatively larger sodium ionic radius with that of
lithium [54]. Synthesis of N-doped graphene foam as anode considerably improves
the performance of sodium-ion batteries [55], as it exhibited an extremely high initial
Fig. 4.12 Chemical route to the iron fluoride-graphene nanocomposites for LIB cathode
materials [10].