Page 175 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 175
Polymer-based nanocomposites 149
[48,191]. However, if the size of the fillers are changed to ellipsoid, percolation can be
obtained at much lower concentration as the fillers will be more easily connected to
form a continuum. Thus, for large-aspect-ratio fillers, percolation threshold changes
inversely with respect to the aspect ratio of the fillers [143].
Table 5.2 presents the relation of percolation threshold with fillers of different
aspect ratios. It can be observed that the percolation threshold depends on the shapes
of the fillers.
BaTiO 3 nanoparticle with the larger diameter (i.e., 700 nm) [199] has a lower per-
colation threshold than the one with the smaller diameter of 100 nm [194,199]. As the
shape varies from nanoparticles to one-dimensional, the percolation thresholds change
to comparatively lower values. For graphenes, the percolation threshold is further
reduced to 0.31 vol% due to its large dimensions as compared with the other fillers
[193]. The polymer with larger polarity, larger viscosity, and lower degree of crystal-
lization causes enhanced percolation thresholds as these properties limit the uniform
distribution of the fillers [198,200,201]. Thus, the percolation phenomenon destroys
the charge-accumulation capability of the polymer nanocomposites and hence makes
them conducting under the influence of an applied electric field. To avoid percolation,
surface modification of nanofillers is imperative [202,203].
5.3 Various types of novel dielectric polymer
nanocomposites
5.3.1 Polyvinylidene flouride
PVDF is the homopolymer of vinylidene fluoride (VDF). It contains 59.4 wt% fluo-
rine and 3 wt% hydrogen atoms with 50%–70% crystallinity. It has glass and melting
temperatures in the range of 40 to 30 and 155–192°C for amorphous and crystal-
line phases, respectively [204]. The Curie temperature is in between 195°C and 197°C
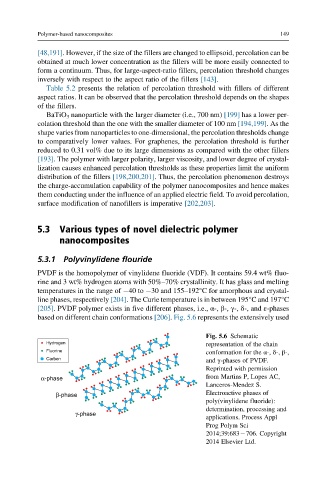
[205]. PVDF polymer exists in five different phases, i.e., α-, β-, γ-, δ-, and ε-phases
based on different chain conformations [206]. Fig. 5.6 represents the extensively used
Fig. 5.6 Schematic
Hydrogen representation of the chain
Fluorine conformation for the α-, δ-, β-,
Carbon and γ-phases of PVDF.
Reprinted with permission
α-phase from Martins P, Lopes AC,
Lanceros-Mendez S.
β-phase Electroactive phases of
poly(vinylidene fluoride):
determination, processing and
γ-phase
applications. Process Appl
Prog Polym Sci
2014;39:683 706. Copyright
2014 Elsevier Ltd.