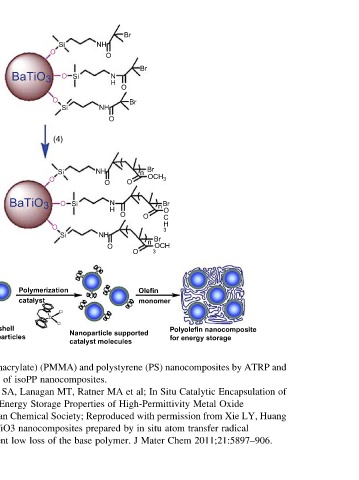
Page 193 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 193
and of Huang
ATRP by Encapsulation LY, radical
Polyolefin nanocomposite for energy storage nanocomposites Catalytic Oxide Metal Xie from transfer 2011;21:5897–906.
OCH 3 Br n O C H 3 Br OCH monomer (PS) Situ High-Permittivity permission atom situ Chem
Br n 3 Olefin In Mater
Br n O al; with in
Br O J
Br O et by
O O polystyrene
N H O N H MA polymer.
O O O Nanoparticle supported of Reproduced
NH NH NH NH catalyst molecules Ratner prepared
Si Si and nanocomposites. Properties base
Si O Si (4) Si O Si Cl Cl (PMMA) MT, Society; the
O O O O Polymerization Zr Storage nanocomposites of
BaTiO 3 BaTiO 3 catalyst isoPP Lanagan Chemical loss low
Core-shell nanoparticles methacrylate) of SA, Energy and inherent
(2) (3) routes DiBenedetto American
m methacrylate)/BaTiO3 the
OH poly(methyl synthesis Dielectric 2010
OH OH P, with
OH BaTiO 3 OH Br exposure to air (O 2 and moisture) high-k the of Tewari Thickness: Copyright materials
HO HO (1) H 2 O 2 , 105 C, 4h O (4) CuBr/PMDETA, MMA for LA, poly(methyl
HO (2) APS, toluene Br Repeat up to five times routes Illustrations Fredin Shell 5154–64. constant
(1) (3) synthesis (B) Z, Li Variable 22: structured dielectric Chemistry.
BaTiO 3 the respectively. from Having 2010; high of
(A) MAO of permission Mater. Core-shell to Society
OH OH Chem. PK. route
OH Illustration Nanoparticles Royal
OH a
OH HO
OH
OH OH OH OH Agglomerated ferroelectric nanoparticles polymerization, with Jiang 2011
HO (A) C,
HO HO HO (B) Nanocomposites.
5.10 Reproduced Core-Shell Wu Copyright
HO polymerization:
Fig. RAFT XY,