Page 269 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 269
Polymer nanocomposite materials in energy storage: Properties and applications 241
[20,23,25,26,28–32]. Synthesis and other issues of the polymer nanocomposites are
discussed in several excellent reviews [33,34].
9.3 Applications of PNCs for energy storage devices
9.3.1 Lithium (Li) ion batteries
Lithium-ion batteries are an important class of electrochemical energy storage
devices. It was manufactured by the Sony Corporation in 1990. Since then, it has gar-
nered immense attention from the scientists and engineers in the process solving the
energy storage problems. Now, it is the most popular rechargeable batteries. The
lithium-ion batteries are very popular and widespread for supply power to mobile
phones, laptops, tablets, and other portable electric devices. They are well suited for
use in vehicles powered by the electricity and for renewable energy systems. They
1
show high energy density (120–170 Wh kg ), light weight, including light weight,
high energy densities, high open-circuit potentials, minimal memory effects, fast
charging, low self-discharge rates, and environmental friendliness compared with
the traditional lead acid, Ni-Mh, or Ni-Cd batteries [35,36]. A lithium-ion battery has
single Li-ion cells connected in series for appropriate voltage or in parallel to increase
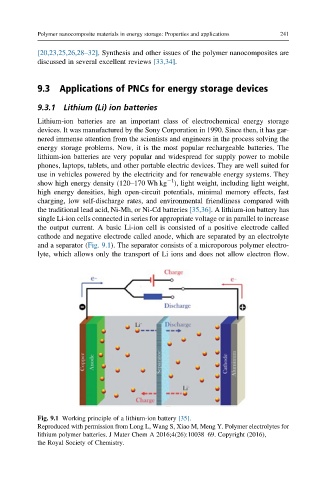
the output current. A basic Li-ion cell is consisted of a positive electrode called
cathode and negative electrode called anode, which are separated by an electrolyte
and a separator (Fig. 9.1). The separator consists of a microporous polymer electro-
lyte, which allows only the transport of Li ions and does not allow electron flow.
Fig. 9.1 Working principle of a lithium-ion battery [35].
Reproduced with permission from Long L, Wang S, Xiao M, Meng Y. Polymer electrolytes for
lithium polymer batteries. J Mater Chem A 2016;4(26):10038–69. Copyright (2016),
the Royal Society of Chemistry.